| You
are here: Home: Meet
The Professors Vol. 1 2003: Case
2
Dr Love: Debu, How often do you
see axillary node recurrence in a woman who’s had axillary
node dissection?
Dr Tripathy: In the published
literature, we see axillary node recurrence in five to ten percent
of women who have had axillary node dissection. There are obviously
different determinants in the risk, such as number of involved
axillary nodes, grade of the tumor, etc. In the Canadian study
of postmastectomy radiation, local recurrence rates were 35 to
40 percent.
As Dr Seidman pointed out, brachial plexopathy and axillary recurrence,
especially with lymphedema, is a very challenging clinical problem.
I would rely on systemic chemotherapy in the palliative setting,
very similar to the way you would treat someone with, for example,
symptomatic pulmonary metastases. As we hear more about this case,
it sounds unlikely that the patient will be a surgical candidate.
The changes in her arm and the possibility of lymphedema and brachial
plexus involvement are concerns.
I disagree with the use of radiation therapy in this case. This
patient probably received 5,000 cGy. It’s hard to conceive
that an additional 2,000 cGy, which would be the absolute most
you could deliver, would help much. In fact, causing an iatrogenic
brachial plexopathy and lymphedema is more likely. I think we’re
left with palliative chemotherapy as our main option.
Dr Love: Dr Argawal, what did
you decide to do?
Dr Argawal: I gave her four
cycles of carboplatin and docetaxel, and she had an excellent response — actually,
I was amazed. The response was so good that she didn’t want
to come for the second cycle — her daughter convinced her
to continue. The mass was much smaller, the node was smaller and
the swelling in her arm had almost completely resolved.
After four cycles of chemotherapy, the surgeon was able to perform
a mastectomy with a part of the pectoral muscle dissected, completely
resecting the mass. Six of lymph nodes out of seven were positive.
She is now completely healed and there is no evidence of disease.
She will also receive additional radiation.
Dr Seidman: If she is very
motivated, she could enter a vaccine clinical trial. Otherwise,
I don’t believe there is anything else you should be doing
for her. I would not continue the chemotherapy because we lack
evidence of its benefit, and the only thing we can be certain of
is additional toxicity in this scenario.
 |
| Case follow-up: |
 |
| • |
Patient received carboplatin and docetaxel weekly
(3 weeks on, 1 week off) x 4; tolerated chemotherapy well but
had several treatment delays due to grade III and IV
hematological toxicity |
| • |
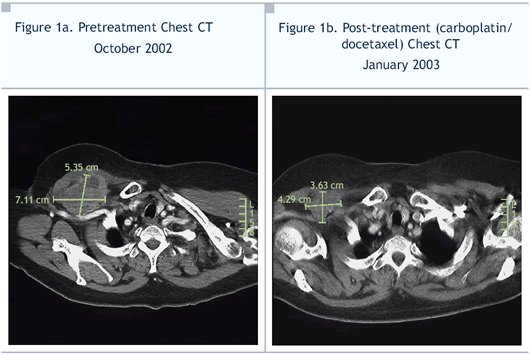
Excellent response; beginning after the first
cycle, mass and lymph nodes smaller on physical exam and CT
(Figure 1b); arm swelling resolved |
| • |
Underwent a mastectomy, partial resection of
pectoralis muscle and axillary node dissection (6/7 nodes positive);
recovered well with no evidence of disease |
| • |
Will receive additional infraclavicular and axillary
radiation |
Dr Tripathy: This patient is
technically NED. You could argue that the likelihood of recurrence
is so high that an additional two cycles of chemotherapy might
delay that recurrence, but it’s hard to believe this would
improve long-term outcome and survivability. This patient has completed
local therapy and has negative margins. I would probably stop at
this point.
Radiation will probably lower the risk of local recurrence, but
the trade-off will be a fair amount of toxicity, especially with
taxanes and I would be concerned about radiation recall phenomena.
Careful shielding and careful attention to detail in port planning
is essential if the patient is going to undergo radiation.
Dr Brooks: How would you follow
a patient like this? My experience is that physical examination
is not very sensitive after these salvage surgical procedures in
the infraclavicular and axillary regions.
Dr Seidman: I agree, but I
would add that routine imaging studies to detect subpalpable disease
radiographically will probably not change the ultimate outcome
for the patient. So, from a cost-effectiveness standpoint, physical
examination and imaging directed by physical examinations and by
symptoms would be indicated.
Dr Brooks: I disagree. I am
a proponent of using tumor markers. I would also use CT scans.
I favor the imaging approach because of the availability of more
tolerable chemotherapeutic agents, such as capecitabine, which
almost deserves its own class. I wouldn’t give her multi-agent
chemotherapy based on a CT scan finding, but I believe that you
can intervene early with capecitabine in a case like this. I think
that you can treat an ER/PR-negative cancer with capecitabine early
on with a good conscience.
Dr Seidman: In a patient like
this with a very high risk of tumor recurrence — particularly
if I knew she had elevated markers at the time of her six-centimeter
mass — I, too, would keep my antenna up by following tumor
markers to intervene early.
Dr Love: Dr Argawal, did this
patient have tumor markers checked?
Dr Argawal: No, I didn’t
follow tumor markers.
Dr Love: Dr Seidman, if, for
example, this woman’s CA 27.29 was very elevated, then dropped
down by a quantum amount after the carboplatin/docetaxel, and dropped
down further after surgery but was still elevated, what would you
do?
Dr Seidman: I would reiterate
Dr Brooks’ comments about capecitabine. I would not treat
a patient with rising tumor markers with any cytotoxic chemotherapy
agent other than capecitabine. I would have a discussion with the
patient about the watch-and-wait approach — beginning therapy
at the onset of symptoms as opposed to the onset of a rise in biochemical
markers. But having said that, once you open the box, it’s
hard to close it.
Dr Tripathy: I agree with Dr.
Argawal’s approach. I would not check tumor markers in the
first place. I don’t know of any reason to use markers, because
it’s not clear that initiating therapy based on marker elevations
helps patients’ outcomes in the long term.
I don’t want to be absolute about it. The fact of the matter
is that I actually have this discussion with patients. I tend to
sway them away from using markers, but I do tell them that there
are very rare potential scenarios in which one, in retrospect,
might say, “I wish I’d used a marker.” For example,
in a patient who develops a fairly rapid complication, such as
a tumor-related brachial plexus problem, by the time you start
them on chemotherapy you cannot alleviate symptoms. You might have
saved, or at least delayed, the onset of that problem.
The risk of using tumor markers in this type of situation is
that we might over-react. We take a patient who perhaps didn’t
need to be exposed to the side effects of chemotherapy for quite
some time, and expose them much earlier because of elevated serum
markers, but we don’t affect their overall clinical course
or their survival. The serum marker problem can cut both ways in
terms of helping you or hurting you.
Dr Love: If you believe that
adjuvant systemic therapy increases survival by treating micrometastatic
disease, why would you not believe that treating Stage IV NED — particularly
when you have an in vivo demonstration of active chemotherapy agents — might
give her a chance of surviving?
Dr Tripathy: If we had more
effective drugs, it’s quite possible that a rising serum
marker, or even a positive PET scan might actually result in an
improvement in outcome. In fact, we know that is the case in lymphoma
and testicular cancer. However, in breast cancer we don’t
have good enough agents to do that at this point.
The second point is that two Italian studies have looked at serum
markers, and there was no difference in outcome between patients
who had them checked versus those who did not. The markers did
predict in which patients cancer was going to recur. A serially
rising serum marker is associated with an 80 percent likelihood
of developing metastases. However, in some cases, these metastases
do not develop radiographically for two or three years. So you
have a patient in limbo, and you can’t do anything. At this
point, there’s no evidence that rising serum markers can
help you treat a patient. They are predictive, and that’s
why the FDA approved them, but they just don’t help with
patient management.
Select publications
|
