| You
are here: Home: Meet
The Professors Vol. 1 2003: Case
6
 |
| • |
1989: 5-cm ER/PR-negative, right breast cancer,
treated with mastectomy, axillary node dissection (negative
nodes); received FAC x 8 chemotherapy and regional radiation
therapy |
| • |
2000: Patient is now in her 60s and asymptomatic;
pleural effusion detected on clinical examination, confirmed
by CT of the chest |
| • |
Bone scan, CBC, liver function studies normal |
| • |
CT of the abdomen and pelvis showed ascites,
enlarged uterus and retroperitonial lymph nodes; ovaries not
visible |
| • |
CA.125 = 20,728 U/mL; CA 27.29 = 355 U/mL; CEA
= 0.8 ng/mL |
| • |
Thoracentesis showed poorly differentiated carcinoma,
most likely an adenocarcinoma but unable to determine whether
the primary tumor was breast or ovarian |
| |
|
 |
| Key discussion points: |
 |
 |
The utility of tumor markers in breast cancer
management |
 |
Differential diagnosis: Metastatic breast cancer
versus advanced ovarian cancer |
 |
Treating a patient with advanced disease and
an unknown site of origin |
 |
Discordance between primary and metastatic hormone
receptors |
 |
Treatment of the postmenopausal patient with
ER/PR-positive metastatic disease |
 |
Dr Pillai: I don’t usually
follow tumor markers in my practice because they may become elevated
three or four months before a clinical diagnosis is made and, in
a stage IV situation, I don’t think that makes a big difference
in the treatment outcome. But in this patient, since I thought
that she might have an ovarian primary, I decided to do markers.
Dr Argawal: A CA.125 of 20,000
U/mL screams ovarian cancer.
Dr Love: Nick, what do we know
about CA.125 in breast cancer?
Dr Robert: CA.125 can be elevated
in all the epithelial cancers, but tumor markers are selected based
on a particular tumor. For example, an elevated CA 27.29 is more
typical of breast cancer. I would agree that this patient’s
tumor marker profile is certainly consistent with ovarian cancer.
Dr Love: Edith, have you ever
heard of a CA.125 as high as 20,000 U/mL in breast cancer? Dr
Perez: No, I haven’t.
Dr Brooks: I do markers on all
my breast cancer patients, specifically CA 27.29, but inadvertently
some patients are also tested for CA.125. I don’t think I’ve
ever seen results in the range of 20,000 U/mL, but I have seen
results in the thousands. I know it’s anecdotal and totally
random, but I’ve ended up with these high numbers in my metastatic
breast cancer patients, ordered sonograms of ovaries and everything
else, but it turns out to be breast cancer.
Dr Aks: The lack of fidelity
of these tumor markers is a genuine issue. I certainly see markedly
elevated levels of CA.125 in patients with non-small cell lung
carcinoma. This particular patient could have colon cancer with
carcinomatosis. You definitely have to go after some tissue and
do a full characterization.
Dr Pillai: I’m old fashioned in that I still try to make
the diagnosis at the bedside. My clinical impression was that this
patient had ovarian cancer because of the pleural effusion, negative
disease in the liver, and the fact that 11 years had passed since
her breast cancer diagnosis. However, I did not want to treat her
without a tissue diagnosis, and I felt the easiest way to obtain
tissue was a thoracentesis. The results showed a poorly differentiated
carcinoma, probably an adenocarcinoma, but the pathologists couldn’t
say whether it was from the breast or ovary.
Dr Perez: In a case like this,
I would take the CT scans to the radiologist and request a pleural
biopsy. To make a diagnosis by cytology alone is very difficult.
It may be easier with a core biopsy.
Dr Robert: There’s a breast
cystic protein stain that could be performed on the primary tumor
and then the fluid to see if it’s positive, but I don’t
know if I’d hang my hat on it. I think the biggest mistake
we can make in a case like this is to assume the patient has recurrent
breast cancer because of her history, and miss a diagnosis. Where
I was trained, we were instructed “if there’s an issue,
get some tissue.” My initial impression is that this woman
has ovarian carcinoma, but we have to establish a tissue diagnosis.
She could have pseudo-Meig’s syndrome, which is malignant
pleural effusion associated with ovarian carcinoma. The bottom
line is you need to get some tissue.
Dr Aks: If the retroperitonial
lymphadenopathy is accessible by CT scan, a fine needle aspiration
may be possible for diagnosis.
Dr Harth: The strong suspicion
is that this patient has ovarian cancer. The next approach would
be to enter her abdomen in some manner to establish a tissue diagnosis,
but I don’t know whether a laparoscopy would be realistic
with such ascites. Therefore, I think we would have to treat her
assuming she has ovarian cancer.
Dr Brooks: This patient has
a large uterus, and they can’t see the ovaries. I think if
she has a gynecologic malignancy, it’s more likely to be
endometrial cancer.
Dr Cohen: I’d do a PET
scan to see if you can identify the ovaries. That would help you
decide whether you needed to perform a laparotomy.
Dr Wilson: I, too, am in favor
of obtaining more tissue. I would recommend approaching a gynecologic
oncologist with this case and discussing the idea of doing a laparoscopic
procedure with the intent of obtaining more tissue. Then, if ovarian
cancer is confirmed, debulking could take place as well.
Dr Pillai: My differential diagnoses for this patient included
ovarian cancer and metastatic breast cancer. If it was ovarian
cancer, it was Stage IV and she was quite symptomatic. I didn’t
think she would be able to go through a laparotomy. I decided to
treat her with a regimen that would work for both breast and ovarian
cancer and then consider interval debulking. I gave her three cycles
of paclitaxel, 175 mg/m2 over three hours, and carboplatin at an
AUC of six — standard doses for ovarian cancer.
The patient had an excellent clinical response. The pleural effusion
and ascites disappeared; the CA.125 dropped to the 500 to 600 U/mL
range; the CA 27.29 dropped about 50 percent; and the CT of the
abdomen and pelvis were normal, except for a smaller but still
bulky uterus. At that point, the gynecologic oncologist consult
recommended continuing chemotherapy. The patient received three
more courses and developed some neuropathy. At the completion of
treatment, the only evidence of disease was a CA.125 of 197 U/mL.
Dr Argawal: This is the kind
of response you see in ovarian cancer.
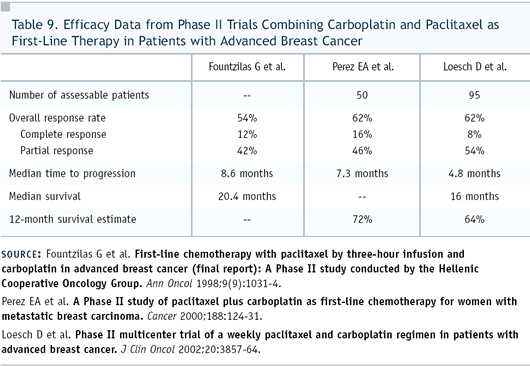
Dr Perez: This is the kind of
response we see with paclitaxel and carboplatin in breast cancer
as well (Table 9), and while it’s great for the patient,
it doesn’t help us in our differential diagnosis. If she’s
tolerating the treatment, I would continue therapy.
Dr Robert: If you assume this
is a metastatic adenocarcinoma, you can give her carboplatin and
a taxane to “cover the waterfront.” But if she really
has an ovarian cancer, the procedure that most gynecologic oncologists
recommend is to debulk the patient. That means not only a laparoscopy
but a laparotomy. You can’t just treat her broadly with chemotherapy.
You still need to know with what you are dealing. When patients
are too sick for surgery, the gynecological oncologists will recommend
starting chemotherapy and will want to see the patients later.
If she has a great response, a laparotomy and debulking procedure
can be done after treatment.
Dr Aks: If you obtain additional
tissue and it shows a poorly differentiated carcinoma or adenocarcinoma,
then the primary site is still unknown. If she has good organ function
and performance status, you could fall back on the so-called Vanderbilt
regimen, which incorporates paclitaxel, carboplatin and etoposide
and covers all the bases.
Dr Firstenberg: I would be
interested in seeing whether she has either ovarian cancer or an
extraovarian papillary carcinomatosis. I am a principal investigator
for a CA.125 antibody trial for which she would be eligible after
she goes into remission.
Dr Pillai: After the chemotherapy, a hysterectomy and bilateral
salpingo-oophorectomy revealed microscopic residual breast cancer
in the uterus, ovaries and fallopian tubes. The hormone receptors
were positive. I believe it’s the same breast cancer, but
I think the methodology for testing has changed.
Dr Perez: The problem of discordance
in hormone receptors between a primary and metastatic site is not
uncommon. A presentation at ASCO addressed this and reported a
discordance rate of almost 25 percent. In our practice, it’s
becoming increasingly common to obtain biopsies when patients develop
metastatic disease and re-test the hormone status. We do this not
only because we’re interested in hormone receptors, but also
to test the tumors for HER2.
Just this week I saw a similar case of a 47-year-old patient
who had breast cancer nine years ago. Originally the tumor was
ER/PR-negative. When it recurred in the pleural fluid, it was ER-positive.
She was then treated with an aromatase inhibitor and the disease
was controlled for one year. Now she has progressive disease, and
we’ll perform another biopsy in order to help us decide how
best to treat her today.
Dr Robert: When Dr Pillai’s
patient was diagnosed 14 years ago, she might have had a charcoal
ligand method used to assess her hormone receptor status. This
older method was associated with a false-negative rate, especially
in premenopausal women, because they were only looking at unoccupied
receptors. The tip-off was that sometimes you would get ER-negative,
PR-positive phenotypes. If an immunohistochemistry had been done
on the original blocks, it might have been positive rather than
negative. Dr Perez’s example, on the other hand, is a bit
more recent, and it might have been tested by immunohistochemistry
the first time around.
Dr Perez: At this point, I would
treat this patient with an aromatase inhibitor rather than tamoxifen
in view of the improved response rate and, in at least one trial,
survival, when compared in the metastatic setting. She has already
experienced toxicity from chemotherapy. It would be easier to maintain
her quality of life with a hormonal therapy than further chemotherapy.
Dr Robert: I would do the same,
and I would use either letrozole or anastrozole. This is a great
case in which the physician treated the patient wisely, and he
continued to ask questions that led to better outcomes for the
patient. Now we have the opportunity to stop chemotherapy because
we know she’s receptor-positive. I agree that the aromatase
inhibitors would be a better choice than tamoxifen, but if she
progresses, a number of hormonal alternatives can be tried. When
necessary, she can be switched from a nonsteroidal aromatase inhibitor
to a steroidal aromatase inhibitor, tamoxifen, fulvestrant, high-dose
estrogens, or androgens.
Dr Pillai: At the time of surgery, the patient was 60 years old,
her performance status was excellent and she had good family support.
I gave her only six courses of chemotherapy preoperatively and
was able to do so within a period of about four months. She still
had an elevated CA.125 of 197 U/mL, so I felt that I should give
her more chemotherapy. I did not want to use a taxane because of
the peripheral neuropathy. I had previously given her doxorubicin,
up to 400 mg/m2, so I was concerned about cardiac toxicity.
I elected to treat her with a protocol first published by Dr
Hainsworth from Vanderbilt called the NFL regimen, which is a combination
of mitoxantrone, 5-FU and leucovorin. It’s an easy protocol
to use, and it doesn’t cause alopecia or peripheral neuropathy.
Myelosuppression is a little more than what you see with a paclitaxel-based
combination. After I gave her six courses, her CA.125 was normal.
I then started her on tamoxifen.
Select publications
|
