You are here: Home: Meet The Professors Vol. 2 2004: Case 1

- Routine annual mammogram revealed changes from the previous year
- Ultrasound revealed three suspicious lumps in the right breast
- Biopsy was positive for infiltrating ductal carcinoma
- Underwent a lumpectomy for two 1.5-centimeter tumors and one 1.0-centimeter tumor
- Tumors were Grade III, ER/PR-positive, HER2-negative
- Sentinel lymph node was negative
- Prior hysterectomy and a history of osteoarthritis, hyperlipidemia and hypertension
 |
| Key discussion points: |
 |
|
Evaluating prognosis for multicentric breast cancer |
|
Use of chemotherapy in patients with ER/PR-positive, node-negative breast cancer |
|
Selection of adjuvant hormonal therapy in postmenopausal women |
|
Use of the Oncotype DX™ breast cancer assay to assist in making decisions about
adjuvant chemotherapy |
 |
DR CARTWRIGHT: This 62-year-old woman underwent a routine annual mammogram which showed a change from the previous year. An ultrasound revealed three suspicious lumps in a cluster in the right breast, and a biopsy was positive. She underwent a lumpectomy and all three lesions were removed. The tumors were Grade III infiltrating ductal adenocarcinoma. Two of these measured 1.5 centimeters and the third was one centimeter. They were all ER/PR-positive and HER2-negative. The sentinel lymph node was negative.
The patient was otherwise in good health. She had undergone a hysterectomy in the past and was on hormone replacement for many years. She had a history of osteoarthritis, hyperlipidemia and hypertension. Interestingly, her mother was a patient of mine who took tamoxifen for five years in the 1980s.
DR LOVE: Peter, what do we do when we have three different primary tumors removed? In a prognostic index, does that count as a 1.5-centimeter tumor or a 3.5-centimeter tumor? Also, can you describe what you think her risk would be?
DR RAVDIN: In terms of a patient who has multiple primary tumors, there isn't an absolute answer. To some degree, the right thing to do is to think of each one as an independent tumor that confers additively to the risk. The first tumor gives her a risk of mortality at 10 years of about 10 percent, being a T1c. In addition, she has another T1c tumor and a T1b tumor. Putting all of that together, she probably has a 20 to 30 percent risk of mortality at 10 years, which is certainly equivalent to having a T2 tumor — this is not a low-risk breast cancer.
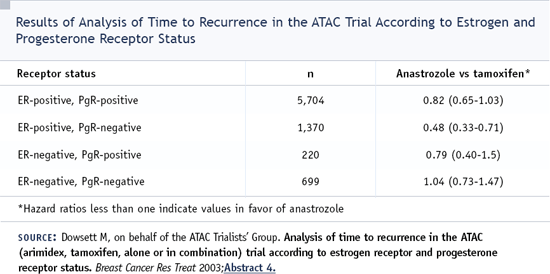
DR LOVE: One of the things we want to get into is the question of which hormonal therapy to recommend for this woman. Gershon is one of the main investigators on the ATAC trial. One of the more interesting recent outcomes of that trial was the 2003 San Antonio presentation by Mitch Dowsett evaluating outcome based on progesterone receptor results. This woman has an ER-positive/PR-positive tumor. Is that relevant in this case?
DR LOCKER: That ATAC presentation was a retrospective subset analysis, and that's a very important consideration. Most of the data presented from ATAC were prospective evaluations. In that retrospective analysis, the women who were ER-positive and PR-positive had an advantage when they received anastrozole compared to tamoxifen. However, in the subset of women who were ER-positive and PR-negative, the benefit was markedly greater for women who were given anastrozole. The hazard ratio was about 0.5 compared to tamoxifen. It's very interesting, but I'm not willing to make decisions based on that data alone.
With regard to this patient, I think the decision to use anastrozole or tamoxifen shouldn't be based on the hormone receptor analysis. This is a woman who is ER/PR-positive, and the data support the superiority of anastrozole over tamoxifen.
DR LOVE: I interviewed Mitch Dowsett in San Antonio after he gave that presentation, and he was speculating that perhaps this subset of patients might be the HER2-positive patients. He seemed to think there was a correlation.
DR LOCKER: There is some suggestion that women who are HER2-positive and ER-positive have lower levels of ER and are more likely to be PR-negative. There is data, particularly from neoadjuvant studies of anastrozole and letrozole, to suggest that anastrozole and letrozole are superior to tamoxifen in that subset of women whose tumors were ER-positive and HER2-positive.
A potential explanation is that in the presence of HER2 overexpression, the coregulators present when tamoxifen binds to the estrogen receptor may actually be those that make tamoxifen seem more estrogenic than antiestrogenic. This is not an issue for women receiving anastrozole because anastrozole doesn't bind to the estrogen receptor and there's little, if any, estrogen to bind to the receptor. If that's the theory, it would be reasonable to assume that in patients with HER2-positive tumors, anastrozole and other aromatase inhibitors might be superior.
DR LOVE: Tom, I know you utilize Peter's ADJUVANT! program (http://www. adjuvantonline.com) and you actually entered this woman's data into the program. Can you tell us what you found?
DR CARTWRIGHT: I did plug her numbers into the ADJUVANT! program, and the chemotherapy regimen I plugged in was for doxorubicin/cyclophosphamide. Eleven out of 100 women benefit from hormonal therapy, and one out of 100 benefit from adding chemotherapy.
DR LOVE: Peter, what are your thoughts on this, and does your model also provide response rates based on tamoxifen versus anastrozole?
DR RAVDIN: The last overview analysis showed that in postmenopausal women — particularly if they were ER-positive — a conventional chemotherapy actually conferred relatively little advantage. In fact, it was slightly less than a 10 percent proportional risk reduction, which is far less than can be expected from hormonal therapy. Hormonal therapy is quite important to these women. The ADJUVANT! program provides firm values for 10 years based on tamoxifen but, of course, we don't have that kind of data for the aromatase inhibitors. If you select an aromatase inhibitor, ADJUVANT! will provide an estimate, based on certain assumptions, from the first four years of the ATAC data, but it has to be clearly stated that we're still looking for long-term efficacy and safety data for the new aromatase inhibitors.
DR LOVE: Before we go on with this case, I'd like to ask Gershon, in general, what's your approach to the postmenopausal woman with ER-positive, node-negative tumors?
DR LOCKER: I agree with Peter that the story with the aromatase inhibitors is still in progress, but we have hard data from the ATAC trial and we will certainly have even more data this summer after another analysis of the data. Virtually all of the patients will have completed therapy for that analysis.
However, so far the absolute difference between tamoxifen and anastrozole, based on four-year data in women such as the one that was presented, is about one to two percent. If you use Peter's ADJUVANT! program, that's the same one to two percent that comes from adding chemotherapy to tamoxifen. So, I tell patients the story for chemotherapy is not clear-cut, and it's not clear-cut in this patient for whom the benefit is only a few percentage points. Anastrozole may actually accomplish the same thing as tamoxifen plus four cycles of AC.
I explain that this is preliminary data based on a median of four years follow-up and making assumptions that nothing else will change. I think it's a reasonable discussion to have with patients, explaining the differential in terms of the benefit from chemotherapy.
DR LOVE: Overall, what fraction of women like this patient receives chemotherapy?
DR LOCKER: In my practice, approximately half of the patients with node-negative disease who do not have any other high-risk factors receive chemotherapy. I do not pressure them to take chemotherapy. I give them a choice and explain the data. Peter's ADJUVANT! program has made it much easier for us because we can provide them with "hard" numbers.
DR LOVE: It's interesting that when we asked more than 700 breast cancer survivors in our patients' perspectives project last year, "If you were in this situation again, would you want to receive AC or CMF chemotherapy for a one percent improvement?" about 55 percent of them said, "Yes," which seems to correlate with Gershon's experience in practice. I want to ask Tom about the next step he discussed with this woman, because there's an interesting twist in this patient's situation.
DR CARTWRIGHT: I actually went one step further and ordered the Oncotype DX™ breast cancer assay, which was just approved by the FDA. It's made by Genomic Health and is based on the information presented at the 2003 San Antonio Breast Cancer Symposium. It costs about $3,400 and it took several weeks to get the insurance company to agree to pay for it. The assay is based on women with Stage I or II, node-negative, ER-positive breast cancer who are treated with tamoxifen. The risk goes from one percent up to almost 50 percent. This patient's recurrence score was 21, which means her risk of relapse at 10 years is approximately 12 or 13 percent. This is almost exactly what was predicted from the ADJUVANT! program.
DR LOVE: The presentation by Dr Paik in San Antonio was one of the most interesting at the last meeting, and everybody came away thinking, "Is this really ready for prime time? Is it worth the money? What does it really mean?" Peter, could you review what the study showed and whether you think it's reasonable to incorporate it into decision-making?
DR RAVDIN: They did a really beautiful piece of work. They developed a 16-gene RT-PCR assay that measures several genes of interest in patients who are node-negative, estrogen receptor-positive. They actually developed and validated the assay in patients who had uniformly received tamoxifen, so the clinical questions the test was developed for are, "For node-negative patients who are estrogen receptor-positive, who will be receiving some form of hormonal therapy, what is their residual risk after that hormonal therapy, and is that residual risk substantial enough to justify the consideration of chemotherapy?"
The test is really paradigm-shifting because for the first time we have a strong multigene test. They developed it in the right way, using banked clinical trial specimens for which we have more than 10 years of follow-up. Their validation study had 675 patients, so they have a large set of patients with substantial follow-up. The test hasn't been immediately adopted by everyone because it still hasn't been published, and in some cases it hasn't been completely worked out. I think it's something that's slowly coming in, and it'll be interesting to see what additional data become available.
DR LOVE: Tom, can you follow up on what happened to this woman?
DR CARTWRIGHT: The Oncotype DX™ assay was useful in this patient. She was at a relatively low risk for recurrence after receiving endocrine therapy. I wouldn't say I could recommend or not recommend chemotherapy to her. I left the decision up to her. Based on this test, she elected not to take chemotherapy. As a result, I put her on anastrozole. Her baseline bone density was normal.
DR LOVE: If this woman had a 20 to 30 percent risk of recurrence, do you think she may have opted for chemotherapy?
DR CARTWRIGHT: Yes. If her risk of recurrence was 20 or 30 percent with the anastrozole, I think she probably would have taken chemotherapy.
DR LOVE: Peter, what would your ADJUVANT! model say in terms of recurrence rate in this woman if she received tamoxifen versus anastrozole?
DR RAVDIN: For a node-negative patient, anastrozole makes the therapy proportionately better by about 10 percent. If her baseline risk had been 20 to 30 percent, the advantage of anastrozole over tamoxifen would be two to three percent, which is pretty close to the results of ATAC.
DR LOVE: I asked Gershon how, in general, he's managing patients like this. Peter, what happens when you see 100 women with node-positive, ER-positive tumors, in terms of how they are treated with hormonal therapy and chemotherapy?
DR RAVDIN: Of women with intermediate-risk, node-negative disease, about two-thirds receive some form of chemotherapy. Universally, if they're estrogen receptor-positive, they receive hormonal therapy. The aromatase inhibitors make that possible, even for some patients for whom tamoxifen is relatively contraindicated. We've all had patients who've had thromboembolic events and other issues, like endometrial cancer.
DR LOVE: In general, what do you usually recommend and what are the patients receiving as hormonal therapy?
DR RAVDIN: Currently, for patients whose disease is equivalent to Stage II node-negative or node-positive, I almost always recommend aromatase inhibitors if they're postmenopausal.
For patients with Stage I estrogen receptor-positive disease except if they're at extremely low risk I still recommend aromatase inhibitors. The few patients for whom I don't recommend an aromatase inhibitor are those who start out with osteoporosis.
DR LOVE: Dr Abel?
DR ABEL: I want to raise a question for which there is no answer. Is the patient going to have a more favorable experience with anastrozole or with tamoxifen followed by letrozole?
The other issue that Dr Locker raised is that the benefit from anastrozole over tamoxifen is equivalent to what might be gained with chemotherapy. If you were to give chemotherapy with anastrozole, you would have a further increment in benefit, which should be considered.
Another issue is that these tumors are close together. If serial sections were done, maybe they were bridged and there's one tumor, or if the molecular genetics were done, do the cells in between really have the changes of malignancy in them already? If so, maybe this really is one tumor rather than multiple tumors. This same risk consideration applies to colon cancers. How do you figure out the risk for multiple tumors?
DR LOVE: That's a great question. Dr Cartwright, where were these tumors in the breast?
DR CARTWRIGHT: All three of the tumors were in the same location. They were in the upper outer quadrant. One thing that's always confusing is, "Are they multicentric or multifocal?" Since they were all ER-positive, HER2-negative, Grade II, we assumed they were multifocal, or just one tumor that had spread through ducts rather than three separate multicentric tumors.
DR LOVE: The implicit question here is, "Is this really a three- or four-centimeter tumor?" Peter, my view is that your data in the ADJUVANT! program is based on actual SEER data, and because there are so few situations in which you have three one-centimeter tumors, I would imagine that we will never have "hard" data on cases like this one. Is that right?
DR RAVDIN: We really don't have exact numerical data about how to make estimates if you have a suggestion of multicentricity in a tumor. What I was suggesting earlier was that for relatively low-risk tumors it's pretty close to additive in terms of risk, so the fact that she had several of these tumors means it is going to push her toward the equivalent of a T2 tumor somewhere greater than two centimeters. She is certainly at a greater risk than a patient with Stage I breast cancer.
DR LOVE: I also want to address Dr Abel's question. Gershon, in the long run, would a patient like this or any patient with an ER-positive tumor who is postmenopausal be better off receiving tamoxifen followed by an aromatase inhibitor? But before we address that, do you think it's reasonable in a situation like this to utilize a test like Oncotype DX™ to make a decision?
DR LOCKER: One of Dr Ravdin's many contributions to breast cancer treatment and research is his insistence on the distinction between prognostic and predictive tests. I've learned a lot from Peter about that. I am convinced that what we see here with this new test is prognostic, but we're using it to determine whether or not to give chemotherapy, and there is no predictive data. We don't know what the benefit of chemotherapy will be in patients who are at high risk based on this prognostic test. That's my concern. I'm convinced that it helps you prognostically, but I'm not ready to use it predictively. I'm curious as to what Peter would say about that.
DR RAVDIN: That is an important question because the test shows the residual risk, and it assumes that chemotherapy has the same probability of being effective or ineffective in those women, as if they had never received tamoxifen.
In NSABP-B-20, half the patients received tamoxifen and the other half received CMF plus tamoxifen. I am curious as to whether or not Genomic Health is going back and evaluating the patients who received both chemotherapy and tamoxifen. That data would give us greater confidence that this test is, in fact, not only identifying people who are at risk for recurrence, but also patients for whom chemotherapy would actually make a positive difference. We don't know that yet. It's being assumed, but it would be nice to see the actual data developed to prove it.
DR LOVE: Gershon, can you address the other question by Dr Abel? In the long run, would patients be better off taking tamoxifen followed by an aromatase inhibitor? I think all the sequencing data has really sensitized us to the time course of recurrence and how much risk a woman is experiencing. How would you respond to that question?
DR LOCKER: The problem is, what are you going to tell the woman who was on tamoxifen in the first five years and relapsed because she wasn't on anastrozole in those first five years, or the women who had a DVT or a PE in those first five years, who wouldn't have had a DVT or a PE had they been on anastrozole?
Admittedly, the woman who doesn't have a fracture occur will be happy, but if she's going to get letrozole later on, she might develop a fracture.
That's with two years of anastrozole versus tamoxifen. The problem is that you can't start from the end of five years of tamoxifen. You have to start looking at events from day one, and there are a group of women who are going to have an adverse effect because they were on tamoxifen and not anastrozole for those five years.
DR LOVE: Dr Weiss?
DR WEISS: You're talking about using letrozole after five years of tamoxifen. Do we have any basis to say we should use five years of anastrozole versus 10 years versus indefinitely?
DR LOCKER: That's a great question. Last week there was a meeting of the ATAC investigators from the United States and Canada, and that was "the" question. What's the optimal duration?
Granted, anastrozole is better than tamoxifen, but for how long should it be administered? We were criticized for not writing that into the trial when the first results came out and, unfortunately, it was a technical issue of not being able to ask the question of five years versus 10 versus a longer duration. The reason we're administering five years of aromatase inhibitors in all of these trials is based on tamoxifen data. It's somewhat naive to assume that the two drugs may require the same duration of therapy. We don't know. It's a great question, and I wish someone would do the trial.
DR LOVE: Peter, what would you do in your practice if a woman who has just finished her fifth year of anastrozole walked into your office? Would you factor in the original risk the way you might as it relates to switching from tamoxifen to an aromatase inhibitor?
DR RAVDIN: At this time, many patients have a substantial risk of recurrence. It's important to point out that late recurrence includes, as defined in these trials, local and distant recurrence and a second primary event. Nonetheless, this risk is substantial — particularly in patients with Stage II disease. It's still even out beyond five years — approximately five percent per year.
A 40 percent reduction in risk is substantial, and these patients could benefit from an aromatase inhibitor. I usually explain that the data isn't as mature as we would like in that we have only three years of data, but it certainly looks positive. Those patients should certainly be offered an aromatase inhibitor, and it should be recommended to them. Patients with Stage II node-negative disease should also receive an aromatase inhibitor. I still have some question in my mind about whether patients who have had Stage I breast cancer in the past should receive an aromatase inhibitor.
DR LOVE: Dr Grabelsky?
DR GRABELSKY: I have computer screens in the exam rooms and I pull up the ADJUVANT! program and show it to patients. It's very nice when you see it in color on the screen. In general, when patients look at that small little sliver of effect chemotherapy has on overall survival, the majority of women say, "I'd rather not go through the side effects of chemotherapy for that small benefit." If you present it as relative risk, more patients would accept it, but when you look at the absolute risk and you see it visually, I think that makes a big impact in how they come to their decisions.
DR LOVE: I have the sense that the culture of oncology has changed in the last few years, and I think a lot of it is from the ADJUVANT! model. Dr Grabelsky, I'm curious if you have changed the way you present this information to women.
DR GRABELSKY: In the past, I would just present the relative risk reduction. Now that we can present the information visually and see how small the absolute difference is, it has probably swayed how I present information and my own feelings about how important it is to recommend adjuvant chemotherapy in postmenopausal patients with node-negative, ER-positive disease.
DR LOCKER: It's laudable that in oncology we discuss absolute benefits. I think about other aspects of medicine. When we say statins save lives, we hear about the relative risk reduction of myocardial infarctions. Has anyone ever told us what the absolute risk reduction is from statins?
We're among the few medical professionals with hard numbers. But, as Dr Weiss said, we have to put the hard numbers into context and say, "Well, an absolute risk reduction of two percent means two people out of 100 won't be here in five or 10 years." However, when you're talking about mortality, a lot of toxicity is acceptable to patients.
DR LOVE: Dr Favis?
DR FAVIS: I think it's almost impossible to be completely unbiased in presenting this information.
I think patients pick up on subtle, subconscious clues, or they may ask you directly what you think. My paradigm has changed. I'm much more likely to suggest chemotherapy and aromatase inhibitors for many of these patients.
The other point is the chemotherapy we gave for Stage I breast cancer five or 10 years ago isn't the same as what we're giving now. I hear more and more researchers on the Breast Cancer Update audio series state that if they're going to give any chemotherapy, they want to give the best chemotherapy. We're widening the distance between the toxicity in both of those situations.
My default treatment is hormone therapy in these patients, unless there's information that the risk is much higher than I would be comfortable with. I've been choosing an aromatase inhibitor almost invariably, except in patients with known bone disease.
DR LOVE: I'm guessing the audio program you might be referring to was the recent one with Craig Henderson, who made the point that generally, when he uses chemotherapy in node-negative patients, he uses a taxane because he wants to use the one that's most effective. Peter, does that logic apply? Can we take the taxane data and apply it in terms of relative risk reduction to a node-negative patient?
DR RAVDIN: I think the answer is that we don't absolutely know because, of course, the trials have been done in patients with node-positive disease. The overview has always suggested that the proportional benefit of adjuvant chemotherapy is at least as large in node-negative disease as in node-positive disease. I think the logical extrapolation is to use the proportional benefit seen in the node-positive patients and carry it forth to the node-negative patients.
The other crucial bit of information — and I've actually discussed this with Craig Henderson — is that proportionately, classic chemotherapies like CMF and AC are less effective in postmenopausal patients than in premenopausal patients. However, an interesting effect was reported in the overview that hasn't been commented on a lot, and that is that when you look at the advantage of anthracycline-based therapy, it's seen equally in premenopausal and postmenopausal patients.
The question I asked Craig Henderson was, "Did the addition of a taxane in trial 9344 have less of an effect in postmenopausal patients than in premenopausal patients?" In fact, it didn't. All of this supports the idea that we should consider using some of the more advanced chemotherapies in postmenopausal women.
DR JOSHUA: I agree with Dr Favis that patients take clues from us when we describe the advantages and disadvantages of therapy. Sometimes, as soon as the word chemotherapy is mentioned, they start shaking their head negatively. Obviously you're going to recommend more hormone therapy. We also take clues from our patients — it's hard to avoid that.
DR DRESDNER: On an opposite note, I've heard a surgeon say to the patient, "I'm sending you to the oncologist for chemotherapy," and when the oncologist tries to recommend hormone therapy, suddenly the patient doesn't understand why we're trying to give them less treatment than recommended by the other expert who just operated on them. It's a strange paradox when a patient actually asks for chemotherapy.
DR JOSHUA: Sometimes it's the other extreme, when the surgeon says, "All you're going to need is hormonal therapy," and then we're trying to explain to them, "No, you're going to need more than hormonal therapy."
DR LOVE: Dr Abel?
DR ABEL: You asked Steve earlier how oncology has changed. I think a very important change is that the oncologists and the patients are better informed as a consequence of having the ADJUVANT! program. Usually the pieces fall into place when you can show the patient the data. The decision becomes much easier and the absolute value demonstrated on the screen is a very effective way to present the data.
Select publications
|
