You are here: Home: Meet The Professors Vol. 2 2004: Case 5

- Mammogram revealed a cluster of microcalcifications in the right breast
- Stereotactic biopsy revealed comedo DCIS
- Re-excision was negative for residual disease
- Underwent lumpectomy, radiation therapy and tamoxifen therapy
- Three years later while on tamoxifen, developed an ER/PR-positive, HER2-negative, 1.0-centimeter, moderately differentiated infiltrating ductal carcinoma in the same breast
 |
| Key discussion points: |
 |
|
Management of the premenopausal patient with an ER-positive tumo |
|
Genetic testing for BRCA-1 and BRCA-2 |
|
Maintaining fertility after ovarian suppression |
|
Use of hormonal therapy for DCIS |
 |
DR GRABELSKY: The patient is a 35-year-old married woman with two children and a strong family history of breast cancer. Her mother had breast cancer, a maternal aunt had ovarian cancer, and a maternal first cousin had bilateral breast cancer. Because of her family history she had early mammograms, one of which showed a cluster of microcalcifications within the right breast. A stereotactic biopsy revealed intraductal carcinoma with comedo necrosis. She underwent re-excision, which revealed no residual intraductal carcinoma. She received radiation therapy and was placed on tamoxifen at that time.
She did well for three years, until routine follow-up mammography revealed a new lesion in a different quadrant of the same breast. She underwent a biopsy, which revealed a 1.0-centimeter, moderately differentiated infiltrating ductal carcinoma. An axillary lymph node dissection revealed 13 negative lymph nodes. Estrogen receptors were 95 percent and progesterone receptors were 90 percent. HER2 was negative by immunohistochemistry.
DR LOVE: Incidentally, was she Jewish, and did the subject of genetic testing come up?
DR GRABELSKY: Yes, and there were some concerns on her part about testing because of insurance issues. Because of the strong family history, she elected to have bilateral mastectomies and an oophorectomy.
DR LOVE: Let's talk about management of this premenopausal woman. Kevin, how would you think this through?
DR FOX: The case would be much more difficult had she not decided to have an oophorectomy. Let's assume for a minute that she chose not to. Here you have a young woman with a relatively low-risk cancer. I think most of us would consider systemic chemotherapy and hormonal therapy as each would contribute a modest amount to reducing her risk of dying of breast cancer.
The choice of chemotherapies is relatively straightforward — it's pretty much whatever you want it to be. I think most of us would probably use AC. Fortunately or unfortunately, a 35-year-old woman does not stand an overwhelmingly high chance of going into menopause with AC, so let's assume she retains her menstrual function, with a tamoxifen-resistant cancer. My own bias — although I've never actually done this — would be to induce ovarian failure with medical oophorectomy and utilize an aromatase inhibitor.
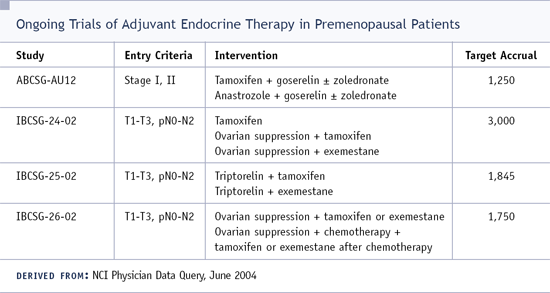
DR ELLEDGE: I think the answer to the question, "Does oophorectomy add to tamoxifen in premenopausal patients?" is still out there. We have a large international trial addressing that very issue, but everything we know about estrogen, breast cancer and the interaction of a ligand with its receptor in an environment of high estrogen would suggest that it would be better to have low amounts of estrogen.
We still need to do a trial to prove that, but I am very nervous when I have a multiple node-positive patient who is premenopausal, ER-positive and not rendered amenorrheic by chemotherapy. I have induced menopause in a very small number of these patients. It has not been more than five in the last five years, but I have done it.
DR LOVE: The other issue is patient attitude. Can you talk more about her thoughts about chemotherapy?
DR GRABELSKY: In addition to being a full-time mother, she is an aerobics instructor and personal trainer. We had a long discussion, and she was very concerned about body image and the side effects of chemotherapy. We talked about what might happen if she remained premenopausal versus undergoing surgical oophorectomy and what the implications were for hormonal therapy versus chemotherapy.
After she underwent the oophorectomy, we again discussed the additional merits of chemotherapy in a one-centimeter, Grade II, strongly ER/PR-positive, HER2-negative tumor. She was fairly adamant about not receiving chemotherapy, and we elected to treat her with anastrozole alone. DR LOVE: Kevin, when we talk about management of premenopausal patients, particularly those who want to have children in the future, your name often comes up for some of the innovative work you've done. Can you update us on that?
DR FOX: Based on some suggestive information from Hodgkin's disease, we utilized ovarian suppression concurrent with chemotherapy in about 30 patients in an attempt to protect their ovaries from the cytotoxic effects of the chemotherapy. The primary intent of this project was to see if we could preserve menstrual function and document how many women retained their ability to have cyclic menses upon the completion of chemotherapy. The secondary intent was, of course, something that is much harder to measure — fertility.
We found that we were able to retain menstrual function in 29 out of 30 patients. It is remarkable that if you give a woman three or six months of leuprolide acetate or goserelin, she will stop having menses and then, almost predictably, resume having them in about six months.
That was fine until we began to look at fertility outcomes over the next few yeas. I found the results very discouraging. Of the 30 patients evaluated, 11 claimed to be actively attempting to become pregnant. Five of these women were able to get pregnant but only two were successful without infertility treatment. Overall, there were six pregnancies but only two successful births.
You can look at this in many ways. First, the number of patients is too small to draw any sweeping conclusions. However, when we saw how difficult it was for these patients to become pregnant, we began to ask whether we were doing them any good. The cooperative groups have taken a larger interest in this project, which I think is good, but in my opinion, continuing it in a single-institution fashion was less than responsible.
The second problem is that the estrogen receptor-positive women, which constituted just over half of our patient cohort, under many circumstances would not take the tamoxifen that was being recommended to them because they intended to become pregnant. In our cohort of 30 patients, six relapsed, four of whom were ER-positive women and had declined tamoxifen. To me, that was an even more troubling aspect of this effort, and we no longer do this outside of a clinical protocol.
DR LOVE: If this woman had decided to undergo genetic testing and was found to be negative or had decided not to have her ovaries taken out, would that have changed your approach to this situation?
DR FOX: I have developed a healthy respect and distrust for BRCA-1 and BRCA-2 in terms of what they mean to patients who are not carriers of identifiable mutations. This woman is Jewish, and her family history hints at something that is genetically driven. If she were not a mutation carrier by current identification, I would not have discouraged her from having prophylactic surgery. As far as her ovarian function, I would have induced menopause medically in order to justify the use of an aromatase inhibitor, which would be her best therapeutic option in the context of tamoxifen resistance.
DR ELLEDGE: I would have strongly recommended that she have a genetic evaluation even if she did not want genetic testing. Our genetics counselor will take an entire family pedigree, put it into a computer, and estimate the probability of a BRCA-1 or BRCA-2 mutation. With this family history, our model would spit out a number that approaches 100 percent in terms of a BRCA-1 or BRCA-2 mutation.
Testing in the Ashkenazi Jewish population is more technically straightforward, and I would have recommended that she have an Ashkenazi Jewish panel performed. I think that it would have been positive, probably for BRCA-2. It doesn't sound like it would have changed anything that she did, but it would allow other family members to make important decisions.
DR GRABELSKY: In regard to Richard's point, she actually did seek genetic counseling and they felt that there was a high likelihood that she would test positive. As soon as her insurance situation stabilizes, she intends to be tested because she has a daughter.
DR ELLEDGE: Just as a practical point, I tell my patients it is illegal to discriminate against a person on the basis of a genetic test either for insurance premiums or for employability.
DR LOVE: Kevin, this case also brings up the issue of hormonal therapy in DCIS, as this woman broke through her treatment with tamoxifen. In this case it was an invasive recurrence, but about half of the recurrences are recurrent DCIS. Can you talk about your approach to hormonal therapy for DCIS and particularly the woman who progresses on tamoxifen?
DR FOX: The current clinical trial of tamoxifen versus anastrozole in DCIS is predicated on the presence of estrogen receptor, so a default position has been taken that tamoxifen or anastrozole cannot possibly work if the patient's DCIS does not express estrogen receptor. Although I think it is intuitively obvious that that should be the case, are mechanisms at play here that guarantee tamoxifen to be worthless in a woman whose DCIS is ER-negative?
DR LOVE: So, you are concerned that maybe there is benefit in women who are ER-negative. The other thing that you might want to comment on, Rich, is that women who are called ER-negative in the community actually did benefit because there were so many false negatives.
DR ELLEDGE: Using Craig Allred's technique as the gold standard and it's been validated to be accurate in a number of clinical trials — there is a 50 percent misclassification for estrogen receptor status in DCIS in his study of 400 patients. Unfortunately, it was a coin flip as to the estrogen receptor status. His evaluation detected no benefit in ER-negative DCIS, which goes along with what we see in invasive breast cancer. The data is not strong in terms of statistical stability, but it's backed up by a lot of biology. It needs to be confirmed in another study, but I'd be surprised if it was wrong.
DR ABEL: How should we handle an ER-negative DCIS case in the community? We probably do not have the luxury of getting repeat testing done through the insurance companies. Shall we assume they are all positive?
DR ELLEDGE: No. I do not think so, because doing so would subject your patient to five years of a drug with significant cost and potential side effects. What you can do is look at the lab where your sample was tested. If it is one of the larger central labs, you can have more confidence. I really get nervous about smaller individual hospitals because that is where the mistakes are made.
Select publications
|
