You are here: Home: Meet The Professors Vol. 3 2004: Case 2

- Presented with an abnormal mammogram
- Underwent lumpectomy with axillary node dissection for 3.2-centimeter tumor and no positive lymph nodes
- Tumor was 60 percent ER-positive, 40 percent PR-positive and HER2-negative by IHC
- Received adjuvant radiation therapy, AC x 4 followed by tamoxifen
 |
| Key discussion points: |
 |
|
Role of Oncotype DXTM assay in clinical practice |
|
Use of the Ravdin Adjuvant! model |
|
Selection of adjuvant chemotherapy for patients with node-negative disease |
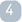 |
Tamoxifen-associated weight gain |
 |
Side effects of adjuvant aromatase inhibitors and tamoxifen |
|
Use of bisphosphonates in postmenopausal patients treated with an aromatase inhibitor |
 |
DR BERRY: The patient was 57 years old and working full time as a sales assistant. She is a rather large lady — approximately 5’8” and 180 pounds. She originally presented with an abnormal mammogram and subsequently went on to have a lumpectomy during which a 3.2-centimeter, T2 tumor was discovered. She also had an axillary dissection that revealed no positive lymph nodes. The tumor’s ER was 60 percent positive, PR was 40 percent positive, and the Ki67 was eight percent. HER2 was negative by immunohistochemistry.
Overall, this woman had a positive attitude and was keen to have breast conservation. She was willing to undergo the rigors of radiation therapy and wanted to do anything she could to reduce her risk of recurrence.
DR LOVE: Adam, in general, how would you have thought through this situation?
DR BRUFSKY: My general approach in postmenopausal women with T2 tumors who are under 65 or 70 years of age is to offer them chemotherapy and an aromatase inhibitor. However, I think this is a case for which you could consider using the Oncotype DX™ test.
DR LOVE: Can you talk a little bit more about that test?
DR BRUFSKY: It is a reverse transcriptase, PCR-based test based on paraffin-embedded tissue. A group in California selected 21 genes as the basis for the test. Expression of this 21-gene set is converted into a recurrence score. This has been validated based on retrospective data from an NSABP study. If your recurrence score is high, then you have about a 30 percent chance of relapse with tamoxifen alone, versus 10 percent if your recurrence score is low. The idea is that you can then offer chemotherapy to women with higher recurrence scores.
DR LOVE: Let’s say a woman fell into the high-risk range because of this assay or her tumor size, what type of chemotherapy would you recommend?
DR BRUFSKY: Generally, in postmenopausal women, I’ve offered anthracycline-based chemotherapy, such as AC x 4 or FEC 100 x 6 in a non-dose-dense fashion.
DR LOVE: Dan, do you use taxanes in patients with node-negative disease and if so, in what situations?
DR BUDMAN: At our institution, we are not convinced that this test, which costs more than $3,000, offers anything over Peter Ravdin’s Adjuvant! program, which is free.
In the New York area, it depends on whom you talk to. Many of the physicians at Memorial believe a continuum exists between node-positive and node-negative disease and are even treating high-risk, node-negative disease off protocol with dose-dense AC T. At our institution, we have been more conservative and are using the AC x 4 regimen. T. At our institution, we have been more conservative and are using the AC x 4 regimen.
Information from thousands of patients indicates that an aromatase inhibitor is good adjuvant therapy for a postmenopausal woman with ER-positive disease. Is there a downside? Sure. But I’m surprised that ASCO has not picked up on this more strongly, because if this were a chemotherapy drug, we’d be jumping up and down and saying, “Look how good it is!”
I am curious to know what the community experience is with the aromatase inhibitors. In my practice, they are exceedingly well tolerated, but a cohort of women has articular complaints and some are severe. I have taken two patients off of an aromatase inhibitor because they just could not tolerate it. Usually, these symptoms are reversible, but quality of life for those patients was poor.
DR LOVE: I’d like to hear from Dr Merkel because I know he had a very interesting point in this regard.
DR MERKEL: When I was enrolling patients in the ATAC trial, patients occasionally complained of aches and pains very much like what was reported. But as soon as the data became available and I started using anastrozole as first-line adjuvant hormonal therapy, I found I was seeing a lot more articular problems than earlier on.
It was only later that I realized I was using it in a different population of patients. When I was enrolling patients in ATAC, my particular referral pattern was to recommend the trial only to women who were not receiving chemotherapy.
Many of the women I have treated with anastrozole in the last two years received it after chemotherapy. Women can develop postchemotherapy arthralgia, typically three to six months after treatment. That is also the time when they finish their radiation therapy, if they’re receiving it, and then their first month or two of anastrozole.
I think some of the aches and pains that I’ve been blaming on anastrozole are actually old chemotherapy-related arthralgias. Anastrozole may not always be at fault, and we need to try to help people through that three- to six-month window in hopes that things will improve.
DR LOVE: Adam, I thought that was a fascinating point. I had not previously heard anybody mention this as a possibility. What are your thoughts about it?
DR BRUFSKY: This is the first time I’ve heard it. I think it is an interesting point. The other point to make is that sometimes the arthralgias appear, at least anecdotally in my practice, to be idiosyncratic to a particular aromatase inhibitor.
DR LOVE: Dan, what are your thoughts on that?
DR BUDMAN: I have not heard of it before either, but I think it is particularly interesting. Also, it may be that the cytotoxics sensitize the joints in some manner that we are unaware of, but I have no biologic mechanism to account for that.
In my practice, if a patient is intolerant of a nonsteroidal aromatase inhibitor I try them on a steroidal one, or vice versa, and hope for the best. I have seen at least two women who went to a rheumatologist, underwent a complete workup and improved when we stopped the aromatase inhibitor.
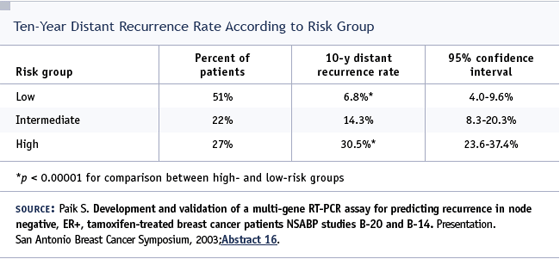
DR LOVE: I want to discuss the issue of bisphosphonates with anastrozole. Adam, we do not have much data, but a presentation from the Austrian group at the 2002 San Antonio Breast Cancer Symposium showed a lot, if not all, of the bone loss associated with an aromatase inhibitor — in this situation an LHRH agonist plus anastrozole — was ameliorated using intravenous bisphosphonates. What are your thoughts about that study and where do you think this is all heading?
DR BRUFSKY: The Austrian study was conducted in premenopausal women who were made postmenopausal by an LHRH agonist. Every woman enrolled in the study received an LHRH agonist, and one half received tamoxifen and the other half received anastrozole. These patients then underwent a second randomization to zoledronic acid or observation, and the women who received zoledronic acid in that trial had less bone loss.
DR LOVE: If you see a woman with nodepositive disease who has osteopenia or even osteoporosis, would you use anastrozole plus a bisphosphonate up front?
DR BRUFSKY: I think it depends on the degree of osteopenia and whether the woman has had fractures in the past. If someone comes in with a T-score of minus two and a half and has not had a fracture, I probably would treat her with a bisphosphonate and an aromatase inhibitor. I would hesitate in a woman with a T-score of minus three who already had a couple of fractures. In my opinion it is really a matter of degree more than anything.
DR LOVE: Dr Shulman?
DR SHULMAN: Dr Brufsky, you mentioned that you would consider using an aromatase inhibitor for a patient who has osteopenia. You also mentioned that you would not give an aromatase inhibitor to a woman who has already had bone fractures. I see a lot of women who have osteoporosis but do not have fractures yet. Right now, I’m staying away from adjuvant aromatase inhibitors in those patients, but what would you suggest?
DR BRUFSKY: It’s a great question. If a woman is already taking a bisphosphonate, calcium and vitamin D, and you follow her closely, I don’t see any reason why you could not give her an aromatase inhibitor as long as you use care. I think it is a reasonable thing to do because, in my opinion, the benefits of aromatase inhibitors over tamoxifen in the adjuvant setting are starting to become substantial. We are now talking about absolute differences of three percent over tamoxifen. I think that is nearing the point at which I am willing to risk a little bit of bone loss.
DR LOVE: Dr Ansari?
DR ANSARI: In a patient with osteopenia or osteoporosis, will you select one aromatase inhibitor over the others because it seems to be less toxic to the bone?
DR LOVE: Adam, at one point we hoped that exemestane might be bone-sparing, but the recent study by Coombes suggested that may not be the case.
DR BRUFSKY: We hoped that because exemestane was steroidal and had some androgenic effects, it somehow would be less osteoporosis-inducing. However, it looks like exemestane does cause bone loss.
DR LOVE: Dr Berry, this woman was diagnosed prior to the ATAC study, what happened with her?
DR BERRY: She received four cycles of AC and, at that time, commenced on tamoxifen. She tolerated it well, but over the next two years she gained 75 pounds. She tried to diet and exercise, but was absolutely disgusted by her weight gain. Then, on routine follow-up examination in May of 2000, she developed obvious hepatomegaly.
DR LOVE: Did she describe a history of eating the same amount and yet gaining 75 pounds?
DR BERRY: No change occured in her lifestyle. She was working full time and was adamant that she had not increased her caloric intake. In fact, she was trying to reduce her eating and increase her exercising. Nonetheless, she steadily gained weight.
DR LOVE: Did any symptoms suggest metastatic disease?

DR BERRY: None whatsoever. She had no arthralgia, did not complain of shortness of breath, and no signs suggested that her performance status was deteriorating — other than the weight gain itself.
Her liver function tests were absolutely normal at that point in time, but on routine physical examination, I was able to appreciate a liver edge, which I had not appreciated before.
DR LOVE: Adam, how would you have thought through this situation?
DR BRUFSKY: In a woman who has had modestly high-risk breast cancer and hepatomegaly, even in the setting of a normal liver function test, I would think this is liver metastases and I would order a CT of the abdomen.
DR LOVE: What are your thoughts about the weight gain?
DR BRUFSKY: I think that we all debate the real cause of weight gain from tamoxifen. A lot of trials enrolled women who were perimenopausal and became postmenopausal, and it is often difficult to determine whether weight gain is attributable to becoming postmenopausal or caused by tamoxifen.
Anecdotally, in my practice I have treated several women who have gained 70 to 100 pounds or more while taking tamoxifen. Most initially weighed over 200 to 300 pounds, but when I’ve discontinued tamoxifen, they have lost the weight.
DR LOVE: Dan, I can remember a panel discussion at the Miami Breast Cancer Conference during which we were talking about tamoxifen and weight gain. Richard Margolese from the NSABP was in the audience and almost jumped out of his chair, “Tamoxifen doesn’t cause weight gain. We have placebo-controlled studies.” Does tamoxifen cause weight gain, Dan?
DR BUDMAN: Good question. The literature tells us that if weight gain occurs, it is usually 10 to 15 pounds at most, so this is particularly unusual. That is not to say that it cannot happen, but other factors may be involved.
DR LOVE: Dr Shulman?
DR SHULMAN: I had a patient who gained about 30 pounds, and when she stopped tamoxifen, she gradually returned to her baseline weight and refused to take the drug anymore.
DR LOVE: Let’s find out what happened to this patient. Dr Berry?
DR BERRY: She had a CT scan that showed pronounced fatty infiltration of the liver. I have seen this before in a number of other patients, but not with such dramatic weight gain.
I suggested that we discontinue the tamoxifen and, because she was only a couple of years out, I ffered her anastrozole as an alternative to reduce her risk of recurrence. She was willing to try that approach and started on anastrozole.
Within two follow-up office visits her liver, clinically, returned to normal and over the course of two years, she lost 60 pounds without a great deal of change in her diet and activity level. I’m convinced it was causally related and have seen a number of other women who have gained 25 to 30 pounds. The majority of women do not gain large amounts of weight on tamoxifen, but it happens often enough that I am not surprised to see it in this setting.
Select publications
|
