You are here: Home: Meet The Professors Vol. 3 2004: Case 5

- Mammographic abnormality and palpable mass in the right breast
- Lumpectomy with SLNB and axillary dissection revealed a 1.5-centimeter, Grade III invasive ductal carcinoma with lymphovascular invasion and one positive lymph node
- ER/PR-negative, HER2-negative by IHC
- Frequency of cell mitosis is 25
- No comorbid illnesses
- Treated with lumpectomy, AC for 4 cycles and radiation therapy without difficulty
 |
| Key discussion points: |
 |
|
Role of adjuvant systemic therapy in healthy elderly patients with high-risk ER/PRnegative, HER2-negative disease |
|
Use of chemotherapy in elderly patients with visceral metastatic disease |
|
Reassessment of tumor HER2 status in patients with metastatic disease |
|
Use of nonprotocol trastuzumab-based therapy in patients with ER/PR-negative, HER2-positive disease |
 |
DR WADE: This 83-year-old woman initially presented in December 2000 with a palpable mass and mammographic abnormality in the right breast. After a needle biopsy, sentinel node evaluation and a subsequent lumpectomy with axillary dissection, she was diagnosed with a 1.5-centimeter, Grade III invasive ductal carcinoma with lymphovascular invasion.
One sentinel node out of a total of nine lymph nodes was involved with ductal carcinoma, but no extracapsular extension was present. She was ER-negative, PR-negative, and HER2-negative by IHC. Parenthetically, she had 25 mitoses per 10 high-power fields on microscopy.
DR LOVE: Was she a healthy 83-year-old? Did she have any comorbid illnesses?
DR WADE: I’d put her performance status somewhere between one and two. She was ambulatory and was reluctant to consider any type of aggressive therapy. Her husband was debilitated and spent most of his time in a wheelchair. Her primary concern was undergoing any kind of treatments that would weaken her and make her unable to care for him.
DR LOVE: Did she have any specific illnesses that were causing her problems?
DR WADE: She didn’t have any major problems with hypertension, cardiovascular disease or diabetes at that time, but she did have arthritis.
DR BUDMAN: Is she cognitively intact? Do you think you could trust this woman with oral medication or would you be uncertain about whether she’s taking it? What medications is she taking? Drug interactions are a major issue that we acknowledge, but really don’t deal with it in oncology because people are on polypharmacies all the time. The husband’s debilitation is obviously a major issue, but does she have any other family support?
DR WADE: Dan, her cognition was fine. She had good memory and understood the issues we were discussing. She was the primary caregiver in the family, and no children were present who could help out. She managed most of the activities of daily living — grocery shopping, housecleaning, dishes and meal preparation.
DR LOVE: What is your assessment of how she might have tolerated different types of chemotherapy?
DR WADE: Usually it’s “put your toe in the water” and find out. Her renal function was adequate, and you might predict that she’d tolerate therapy okay, with the caveat that some patients beginning cytotoxic therapy — particularly agents associated with a lot of mucositis — will have a lot of secondary problems, and you may need to back out quickly.
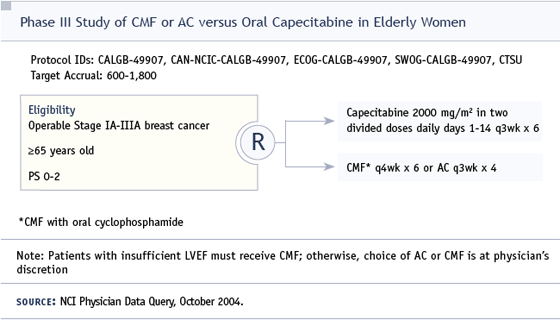
DR LOVE: In this woman, who has ERnegative, node-positive disease, chemotherapy is an issue that must be considered, which was part of the rationale for Hyman Muss to develop the CALGB trial comparing capecitabine to either CA or CMF. That trial has just begun. Dan, how would you have thought through whether to use chemotherapy and, if so, what type?
DR BUDMAN: I would have taken a step back first. Peter Ravdin’s Adjuvant! program is really superb, and I use it all the time. At the last ASCO meeting it was nice to see that the people in British Columbia Cancer Registry actually validated Adjuvant! with 10,000 patients, and it was within one percent.
The only area in which it wasn’t particularly accurate was for patients under 35 years old, which is obviously not a concern in this patient. I would plug this patient’s information into Peter’s program to obtain an idea of what type of benefits we’re considering in a woman who has major social responsibilities and no support structure?
DR LOVE: Dr Wade, after you explained the situation to her, do you think she would have been comfortable receiving no adjuvant therapy?
DR WADE: We discussed no adjuvant systemic therapy. She was torn between the lack of toxicity with no therapy weighed against the concern she would become ill and be unable to care for her husband.
DR LOVE: Adam, would you have used chemotherapy in this situation? If so, what type?
DR BRUFSKY: I would not use chemotherapy in this situation. We actually plugged her information into the Adjuvant! Palm Pilot program. From standard CMF-based chemotherapy, she would probably have a benefit of approximately 1.4 percent due to competing causes of mortality.
If she was seriously considering chemotherapy, and said “I really want to do everything possible, even if it’s a one percent benefit for my relapse rate in five years,” I would consider the capecitabine trial or a mild regimen such as CMF.
DR LOVE: If the capecitabine trial data were available and demonstrated equivalent benefit for CMF and AC, would you use capecitabine or CMF?
DR BRUFSKY: If the data were available, I would use capecitabine.
DR LOVE: Dan?
DR BUDMAN: The CALGB breast community was very interested in this study, perhaps not in an 83-year-old patient, but in patients up to 75 years old with a little more social support.
The problem is we’re waiting for the data. Capecitabine is very attractive. I believe this is an important study, but we don’t have the answer yet.
DR LOVE: Dan, how do you approach dosing with capecitabine?
DR BUDMAN: I look at it a little differently because we know that women have slightly lower DPD levels than men. In fact, when we did a Phase I study, our dose of capecitabine was lower than the dose utilized by the Europeans, because they were enrolling male patients with rectal cancer in their study, whereas we enrolled only breast cancer patients in our study.
We also know that capecitabine becomes more toxic if you have renal insufficiency. As these patients age, they develop end organ dysfunction, so we don’t know whether a dose-response curve really exists. The suggestion from retrospective data, mainly from Joyce O’Shaughnessy, is that the dose-response curve is relatively flat.
Even at 50 percent of the initial dose that she used in the metastatic setting, patients responded. I’d “chicken out” in this case. In an elderly lady, I would probably start at 1,500 mg/m2 per day. If any toxicity occured, I would consider reducing the dose.
DR LOVE: Let’s find out what happened to this patient, at least at that point. Can you talk about your conversation and what ended up happening, Dr Wade?
DR WADE: I discussed the competing issues with her. Would she tolerate therapy? Would it tear her down, or would her disease catch up with her? Keeping in mind that she had a Grade III malignancy, we even discussed whether or not the addition of a taxane would add any additional benefit for her, because she had node-positive disease.
We eventually decided to try one dose of AC and see how she did. She tolerated it with practically no side effects. She went on to receive four doses of AC and then breast irradiation.
The question of performing lumpectomy versus mastectomy was appropriate because she had to drive about 40 miles to receive radiation therapy. However, I saw her after those decisions were already made, and she made it clear to the surgeon that, if possible, she wanted to have breastconserving therapy. She went through her radiation therapy without difficulty and drove herself back and forth for six weeks.
DR LOVE: Overall, how did she tolerate the AC?
DR WADE: She tolerated the AC fine and received therapy on schedule without dose reduction or mucositis.
DR LOVE: Was she able to continue to take care of her husband?
DR WADE: Yes.
DR LOVE: Dan, the dose-dense CALGB trial 9741 has resulted in a lot of physicians using dose-dense AC 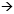 T every two weeks. Interestingly, the node-negative Intergroup trial that followed the report of 9741 switched to using dose-dense AC every two weeks. What are your thoughts about that strategy? Would you have considered it in this woman if she wanted to receive AC? T every two weeks. Interestingly, the node-negative Intergroup trial that followed the report of 9741 switched to using dose-dense AC every two weeks. What are your thoughts about that strategy? Would you have considered it in this woman if she wanted to receive AC?
DR BUDMAN: One of the concerns around the country is that the taxanes, especially paclitaxel, seem to be more efficacious if given more frequently. Andy Seidman’s study in the metastatic setting demonstrated paclitaxel administered weekly was superior to the every three-week schedule. My suspicion is that part of the difference is due to the taxane scheduling.
An Intergroup study that is closed to accrual evaluated every three-week versus weekly paclitaxel and docetaxel. Joe Sparano informed me that, hopefully, the data will be mature enough by next year’s ASCO. Hopefully, that will also add to our knowledge about how to use these drugs.
I am a little wary of giving dose-dense chemotherapy to the elderly. Most of the patients in the CALGB trials are under 65 years old, and the average age in CALGB- 9741 was 55 years.
DR LOVE: Dr Dragon, how do you use growth factors in the older patient? Do you use dose-dense chemotherapy, and would you have considered dose-dense AC in this woman?
DR DRAGON: With regard to using growth factors, much of what I’ve done in my practice is based on the CHOP experience in elderly patients. The only way to effectively and safely give CHOP in patients over the age of 65 has generally been with the regular use of growth factors, so when I treat older patients with AC, I typically administer growth factor support.
Let me play the devil’s advocate. In the meta-analysis, very little data exists for treating patients over the age of 70 years. In patients over the age of 50, the benefits of adjuvant chemotherapy are often quite marginal, and they seem to be even further attenuated in patients over the age of 60 and, we presume, over the age of 70. I can’t explain why this occurs, but nonetheless we have to recognize that a diminution in the effectiveness of adjuvant chemotherapy occurs in elderly patients with breast cancer.
In a patient like this, I wouldn’t ask the question, “Can we treat her?” I’d ask, “Should we treat her?” Frankly, I’d be reluctant to treat this patient with chemotherapy.
DR LOVE: Would you discuss the option with the patient?
DR DRAGON: No, I would not, and my habit is to discuss all the options with patients. Postmenopausal patients will have a two to three percent disease-free survival advantage from chemotherapy. In my experience, the older the patient, the less they want to hear and the more confused they are when they hear the actual statistics. They just want to know, “What should I do, Doctor?”
I would be reluctant to treat this patient, not because I don’t think we can do it safely, but because I’m just not sure we can justify the effort and the utilization of resources.
DR LOVE: We are about to make this a little more complicated, so let’s take it to the next point in this case.
DR WADE: She completed four cycles of AC without difficulty and had breast radiation therapy.
We followed her clinically and I obtained routine annual chest X-rays. She reported some fatigue and back pain. In February 2003, her chest X-ray showed the new appearance of multiple pulmonary nodules. Subsequent imaging showed “plus-minus” for bone metastases but demonstrated the presence of hepatic metastases. We performed a biopsy of her liver, which demonstrated a small area of adenocarcinoma consistent with the original primary tumor.
DR LOVE: What was her functioning at that point?
DR WADE: She was functioning well and was still providing most of the support in the household. She was still driving, able to move around the home and do the cleaning, laundry, etcetera.
DR LOVE: Now she had the experience of having gone through chemotherapy. When you began discussions with her, what was her attitude about the possibility of being re-treated?
DR WADE: Now that she was facing visceral metastatic disease, she wanted to stay alive as long as possible. She did not want just comfort measures only — she wanted active therapy.
DR LOVE: Adam, it becomes more difficult.
DR BRUFSKY: Well, in this case, she is clearly indicating she wants something done. Could she come in for weekly therapy?
DR WADE: She’s close to one of our satellite offices, so we could see her weekly.
DR BRUFSKY: Given her ability to come in weekly, the choices I would probably present are a weekly taxane, either paclitaxel or docetaxel, which would be my first choice if she could tolerate it. The alternative would be capecitabine.
DR LOVE: Dan, what are your thoughts?
DR BUDMAN: Well, I would tend to agree that the database of evidence supports offering an oral fluoropyrimidine, such as capecitabine, or a taxane. In regard to the weekly taxane data, I would differ a little, in that docetaxel offers no real advantage for the weekly over the every three-week schedule, and other problems occur with the nails and the eyes. I would choose weekly paclitaxel, with the hope that some of the water-soluble paclitaxel agents become available soon and will be less toxic.
DR LOVE: If she was treated with weekly paclitaxel and didn’t respond, or progressed, what would be your next therapeutic maneuver?
DR BUDMAN: I could just as easily give her capecitabine first and save the taxane for later treatment. Treatment in this situation is really for palliation and quality of life.
DR LOVE: Adam, what would you do in the same situation, except the patient is 37 years old?
DR BRUFSKY: In a younger patient with visceral disease, I would favor combination therapy. Several regimens are available. The latest regimen — gemcitabine/paclitaxel — was presented at ASCO this year. You could also utilize capecitabine/paclitaxel. Usually I’m a big believer in sequential single agents, but in a patient who has an impending visceral crisis I would vote for a combination therapy.
DR LOVE: Dan, same situation; she’s 37 years old.
DR BUDMAN: I totally agree. I would administer combination chemotherapy to cytoreduce and then, if a response occurs, I would switch to a single agent for quality of life because we know we’re not changing survival.
DR LOVE: Which combination would you use and how would you handle switching to a single agent?
DR BUDMAN: For a response in this setting after failing anthracyclines, the best data right now would be for a combination of docetaxel and capecitabine, and then maintaining her on an oral agent such as capecitabine.
DR LOVE: So, you started off with the docetaxel/capecitabine and after three or four cycles, she had a partial response. At that point, do you switch?
DR BUDMAN: The problem with Joyce O’Shaughnessy’s study is the early death rate on the single-agent arm, which she didn’t correlate with tumor bulk. Most of us are suspicious that visceral crisis is a measure of high tumor bulk, and you want to reduce it as quickly as you can to prevent endorgan damage. You may not have time to use another single agent in that circumstance, which is very different than chest wall or soft tissue disease for which we have plenty of time to play with the drugs.
DR LOVE: Joyce actually talked to me about this strategy of starting with docetaxel and capecitabine, then going on to maintenance capecitabine — a similar strategy to starting out with chemotherapy and switching to hormonal therapy. Is that something that you’ve done in your practice, Adam?
DR BRUFSKY: Yes, we’ve done that. Generally, we’ll administer four to six cycles of combination chemotherapy and then stop or maintain them on single-agent chemotherapy.
DR STEINECKER: Was her ER/PR and HER2 rechecked at the liver biopsy?
DR LOVE: That’s a good question. Dr Wade, can you follow up with what actually happened with this woman?
DR WADE: We didn’t have enough tissue from the liver biopsy to go back and recheck those things. Because her disease appeared to be recurring in such an aggressive fashion, I had the breast tumor sent for FISH analysis.
DR LOVE: What was her original IHC score?
DR WADE: Zero.
DR LOVE: Okay, so you “FISHed” it anyhow?
DR WADE: Yes, and it turned out that she had gene amplification — 5.8 copies — so she was FISH-positive.
DR LOVE: Then what happened?
DR WADE: I met with her and told her that we could try single-agent trastuzumab as an option. It would have relatively little toxicity, and data demonstrated a reasonable response rate. If it worked, it would allow us to avoid cytotoxic therapy for awhile.
DR LOVE: When we talked on the phone, I asked the same question that Dr Steinecker asked: “Did you retest the ER status?” You told me you had not done that.
DR WADE: That’s correct.
DR LOVE: Adam, what are your thoughts about this patient?
DR BRUFSKY: We know from the original pivotal trial of trastuzumab that about 10 percent of women whose tumors are scored as IHC zero or one-plus will test FISHpositive, and obviously she is one of those women.
A lot of discrepancy and discordance exist between community laboratories and central laboratories in IHC testing. I’m assuming this was done in a community laboratory?
DR WADE: The hospital where she actually had the surgery done was not where the test was performed. It was sent to Memrial Medical Center in Springfield, which is a fairly large institution with 800 beds and the pathology team for the medical school has a fair amount of experience.

DR BRUFSKY: So this is probably one of those 10 percent who are really not expressing the protein, but actually have gene amplification.
For this patient, single-agent trastuzumab is a very reasonable therapy. In the Phase II study conducted by Chuck Vogel, the response rate was approximately 26 to 30 percent with trastuzumab monotherapy. I would consider trastuzumab and vinorelbine in someone this old who wanted therapy. A lot of very good Phase II experience with the combination exists, and response rates occur in 60 to 70 percent of patients. That’s probably what I would offer this woman.
DR LOVE: Adam, when you were discussing adjuvant therapy with her, would you have considered treating her with trastuzumab if you had known she was HER2-positive?
DR BRUFSKY: No, I would not. At the time her adjuvant therapy was selected, Chuck Vogel’s monotherapy data wasn’t available. In addition, cardiomyopathy from adjuvant trastuzumab clearly occurs, and that’s causing me to hesitate about using trastuzumab off protocol in the adjuvant setting.
DR LOVE: Dan, in the metastatic setting, now that you know she has a FISH-positive tumor, how would you have thought through her therapy? Also, would you have considered trastuzumab in the adjuvant setting if you had known she was FISH-positive?
DR BUDMAN: No data in the adjuvant setting exists, and we’re waiting for Edith Perez’s study, which I believe is going to close in the next six months, to at least give us some early data.
Data was presented on several thousand patients who had IHC compared to FISH, and for patients with an IHC of zero, two to three percent had FISH-positive tumors, so this patient is unusual.
In her study, Edith has noted frequent discordance between the local and central laboratory HER2 results. It’s worrisome, because we’re obviously dependent upon our local laboratories.
I would approach therapeutic decisionmaking in the same way as Dr Brufsky. Vinorelbine administered at a reasonable dose is well tolerated in the elderly, and the Farber group has a lot of data suggesting synergism between vinorelbine and trastuzumab.
This patient has significant visceral disease, which I’d like to try to down-stage and then maintain her on trastuzumab monotherapy for quality of life.
DR LOVE: Would you bring us up to date on this woman, Dr Wade?
DR WADE: One advantage of trastuzumab alone is that you can administer it on a 21-day schedule, which we did. After three cycles, we repeated CTs and she had a partial response in her liver and lung. We checked it again after another three cycles, and she was starting to progress in the same locations.
After discussing the various options, including one that is not fully rooted in the literature, capecitabine was added to trastuzumab in June 2003; she began treatment for metastatic disease in February 2003. She responded again and continued on a threeweek schedule until January 2004, when she had progression in the liver and breast pain on the treated side.
Capecitabine was discontinued, and she was treated with weekly paclitaxel plus trastuzumab from January until June 2004. She had a partial response after two cycles and was re-evaluated with the intent to stop the paclitaxel and continue on trastuzumab alone.
Over the last two months, she has developed increasing numbness in her fingers and toes, and has more and more trouble moving around. She has to use a walker and needs to position furniture around the house so she can lean on it to move around the house. She didn’t want to use a walker or a cane in her home. After she finished the last round of therapy and came in, she needed to use a wheelchair. I thought, “I’ve really done it with the paclitaxel peripheral neuropathy.”
Computed tomography of her head revealed multiple cerebral metastases, while chest and hepatic CTs still showed a partial response.
DR LOVE: What are you thinking at this point? Does she have any neurologic symptoms centrally, as opposed to peripheral neuropathy?
DR WADE: She has weakness in her right arm to the extent that she can’t write anymore.
DR LOVE: Did you start her on steroids and radiation therapy?
DR WADE: She started on steroids and after 24 hours, her right arm improved. She’s undergoing whole brain irradiation, and when she completes it, we’ll probably sit down and talk about her entering hospice.
DR LOVE: Any comments, Adam?
DR BRUFSKY: As far as we know, trastuzumab doesn’t cross the blood-brain barrier. Based on data from a number of abstracts presented at ASCO over the last year or two, approximately half of women who are on trastuzumab-containing therapies will relapse with brain metastases.
I don’t know what my colleagues do, but in my practice I’ve started to order screening head CTs every six months in patients with HER2-positive metastatic disease.
DR LOVE: Dan, it sounds like this woman had a response to capecitabine and trastuzumab. Initially, there was discussion about Dennis Slamon’s work in vitro. Now I’m hearing more people talking about that combination. What are your thoughts about that?
DR BUDMAN: Two years ago we presented data in San Antonio, but depending on which cell lines are utilized and the model, different results are obtained. In our data we were able to show at least synergism in vitro between trastuzumab and capecitabine. The Japanese also demonstrated synergism in a xenograft model. Does that prove anything in humans? Of course not.
Perhaps the whole “kick” you saw was capecitabine, and she didn’t need the trastuzumab at all. This is a very frustrating area in clinical practice because I’m always on the fence when I have a patient who’s been on trastuzumab and failed that regimen. What should I do? Should I still give it to them or should I stop it? No guidelines are available to tell us what to do.
DR LOVE: Dr Wade, how did she tolerate the capecitabine?
DR WADE: Very well. She had minimal problems with tenderness in her hands and feet.
DR LOVE: Did you ever have to dose-reduce?
DR WADE: No.
DR LOVE: Dr Berry?
DR BERRY: Did this patient truly have a visceral crisis simply because she had liver, lung and bone metastases? If she was FISHpositive, it would not be unreasonable to deliver trastuzumab as a single agent because the response rates are in excess of 30 percent. My experience with paclitaxel in the elderly, no matter how it is administered, has shown a high incidence of neurotoxicity.
If I experience a lack of response with trastuzumab, I add vinorelbine. Assuming eventual resistance, I would consider an equally less toxic agent, such as gemcitabine. I’m not sure I would use systemic chemotherapy by itself in a patient who’s HER2-positive. If she remained FISHnegative, you have little recourse to using the capecitabine-based regimen.
DR LOVE: I set up the geriatrics program at the University of Miami in the mid-1980s, and one of the things that Hyman Muss has talked about over the years is the myth of aging and the importance of being careful generalizing about patients who are in their eighties. This woman, as you said from the beginning, decided she wanted a lumpectomy and was willing to drive 40 miles for radiation therapy. This is not the type of personality that most people think about when they hear about 87-year-old patients.
DR STEINECKER: I don’t know if anybody has ever tried temozolomide with trastuzumab, but it might be worthwhile if your patient wanted to continue therapy. I’ve had some patients who have been long-term survivors, even with brain metastases and breast cancer. I know it’s going to be hard if she’s elderly and weak, but that might be one consideration.
DR LOVE: That’s a great thought. Adam?
DR BRUFSKY: Less enthusiasm exists for temozolomide, because a Canadian Phase II trial that used temozolomide for brain metastases and breast cancer didn’t have good results. Temozolomide didn’t add much benefit, so little enthusiasm exists to repeat that study in the United States.
However, I think the combination of some sort of agent that penetrates the central nervous system in HER2-positive disease is very important. We have to find that agent. Is temozolomide the right agent? I take an aggressive approach to brain metastases, especially in patients with HER2-positive disease who have their visceral disease under control. I do whole-brain radiation and gamma knife radiosurgery, and in a few cases I have used temozolomide in that setting and have had mixed results, but it is of interest.
Select publications
|
