You are here: Home: Meet The Professors Vol. 3 Issue 6 2005: Case 1

Edited excerpts from the discussion:
DR FALLON: This woman presented in March
2003 at the age of 40 with a palpable tumor
in the right breast. The mammogram showed
several areas of suspicion, and the ultrasound
showed one large mass and possibly
a second mass. On stereotactic biopsy, she
had high-grade, invasive ductal, ER-negative,
PR-negative, strongly HER2-positive
breast cancer. At mastectomy, four separate
areas of invasive tumor were found,
measuring 0.6 centimeter, 0.9 centimeter,
one centimeter, and another measuring 2.4
centimeters with a margin less than two
millimeters deep. Two out of 19 lymph nodes
were positive, and no extracapsular spread
was observed on the lymph nodes.
The patient underwent modified radical
mastectomy and immediate implant reconstruction.
She enrolled on NSABP-B-31 and
had hoped to receive trastuzumab but in
fact was randomly assigned to receive AC
times four followed by paclitaxel times four,
without trastuzumab.
She received chest wall radiation therapy
because of the close margin. By the end of
the trial, she was doing extremely well. She
finished her doctorate in special education
and got a new job as principal of a
grammar school. This summer she climbed
the Adirondacks with her sister, which was
about a three-week hike.
She called me in June to ask whether she
could receive trastuzumab at this point, and
I told her I would look into it. She didn’t
want to do anything until she got back in
August, and by that time the NSABP told us
they would allow trastuzumab only to those
who were randomly assigned after April
2004, but she was randomly assigned in
April of 2003.
DR LOVE: How long has it been since she
completed chemotherapy?
DR FALLON: Approximately two and a half
years.
DR LOVE: Eric, would you recommend trastuzumab
off study for this patient?
DR WINER: I would be inclined not to
administer trastuzumab at the moment for
two reasons. One is that whether trastuzumab
works as a single agent when given
two years after diagnosis is unknown.
Second, whatever her risk of recurrence was
in 2003, she has a somewhat lower risk of
recurrence, perhaps a substantially lower
risk, two years later.
Unlike ER-positive breast cancer, in which
events are strung out over the course of 10
to 15 years, in HER2-positive breast cancer
most of the events occur in the first five
years and a lot of them occur in the first
couple of years. That is part of the reason
why, in each of these studies, we saw a
dramatic benefit early on, even in the first
year (Perez 2005b; Piccart-Gebhart 2005;
Romond 2005).
DR LOVE: What would you say if this patient
asked you what her risk of recurrence was
from this point on?
DR WINER: I don’t think we can give her a
hard number, but I believe it’s more than 10
percent.
DR LOVE: Dr Fallon, do you think if this
woman knew she had a 10 percent risk of
recurrence, she would want therapy despite
the potential risks?
DR FALLON: She’s asking for the therapy,
but she’s very philosophical and she agreed
that if we don’t know how much benefit she would gain at this point in time, she may
just wait and use it if she needs it.
DR WINER: Initially, this patient’s risk of
recurrence was in the range of 50 percent
in the absence of any therapy. AC followed
by paclitaxel decreased that by about 50
percent, so at the end of therapy, her recurrence
risk was approximately 25 percent.
DR LOVE: Over two years have passed since
this patient completed chemotherapy. What
is risk of recurrence now?
DR WINER: I suspect that at least a third
of all of the events occur in the first couple
of years, but none of us can give you an
exact answer, partially because we just don’t
have a great database on patients with
HER2-positive disease treated only with AC
followed by paclitaxel.
DR LOVE: How would you respond if the
patient asked, “I know we don’t have any
data, but do you believe my relapse rate
would be decreased if I take trastuzumab
now?”
DR WINER: I think it’s unknown, but if
you invoke the HERA data (Piccart-Gebhart
2005), you would conclude that single-agent
trastuzumab given immediately after the
completion of chemotherapy is a very effective
approach (1.1).
If you look at Edith Perez’s data from the
N9831 study, there are questions about how
much benefit a patient would receive from
single-agent trastuzumab (Perez 2005).
Additionally, some small risk of cardiac
toxicity clearly exists, in addition to the
unknown risks of long-term trastuzumab
after an anthracycline-based regimen.
DR LOVE: This patient is 42 years old.
Assuming she has a normal ejection fraction,
what is her risk of cardiomyopathy or
cardiac failure with trastuzumab?
DR WINER: It’s in the range of one to four
percent.
DR FALLON: I think it’s important to remind
patients that MUGAs don’t prevent cardiac
toxicity. I did one on a patient whom I
started on trastuzumab after doxorubicin
and cyclophosphamide for locally advanced
disease. After day one of trastuzumab and
day two of paclitaxel, she presented with
an unmeasurable ejection fraction. She had
received full-dose doxorubicin, but she had
a normal MUGA at the beginning and end of
doxorubicin and then she suddenly developed
acute cardiotoxicity.
When she then came back with metastatic
disease, I treated her with docetaxel alone.
Since her cardiac condition had reversed,
eventually I was tempted to administer
trastuzumab again. I administered it to her
with vinorelbine, but her ejection fraction
again decreased, so now she’s on vinorelbine
alone.
So while I do think cardiac dysfuction with
trastuzumab is more reversible than doxorubicin-
induced cardiac failure, it’s not a walk
in the park. I do think it’s reasonable with
the patient I presented today. But she wants
to be active — that’s a big part of her lifestyle
— so it’s a balance of unknown benefit
against possible risk.

DR LOVE: Eric, what are your thoughts about
the use of trastuzumab without chemotherapy
in the adjuvant setting for patients
in whom you are reluctant to use chemotherapy,
either because of age or comorbidities?
DR WINER: I wouldn’t do it. As tempted as
we all might be, there simply aren’t data
from any of the trials. That said, I think that
a regimen of some chemotherapy followed
by trastuzumab, based on HERA, is justifiable
(Piccart-Gebhart 2005). In this woman,
I think the key issue is making sure that
she’s comfortable with the decision. I try
not to draw a line in the sand and say,
“We can’t cross this line” but to really help
patients make the decision with me. The
fact is that this patient could have a recurrence
no matter what you do. If she receives
trastuzumab, she could develop heart
failure. You just can’t predict all of this.
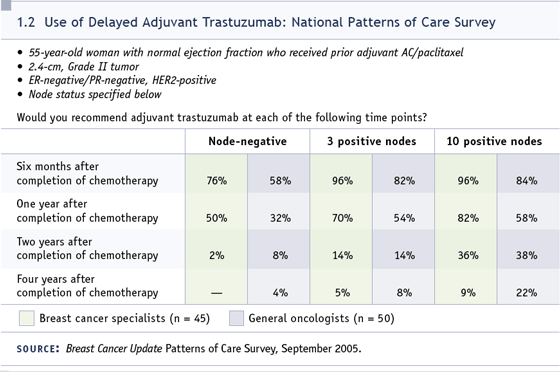
DR LOVE: Kevin, would you administer
delayed adjuvant trastuzumab to this
patient (1.2)?
DR FOX: I find that when I’ve been
confronted with these agonizing situations
that present an impossible calculation
of risk and benefit, I have at times taken
the “oh, what the heck” approach. I have
administered delayed trastuzumab a couple
of times, but I would not if I felt the risk
of recurrence were so absurdly small that I
couldn’t justify it — for example, the onecentimeter,
HER2-positive cancer that was
treated five years ago. That would be an
easier case to decide than this one. I have
to confess I’ve been a little bit “fast and
loose” with delayed trastuzumab.
DR LOVE: Eric, in the data from the BCIRG
006 study, we see that patients on the TCH
[docetaxel/carboplatin or cisplatin/trastuzumab]
arm had a 39 percent reduction in
their relapse rate, whereas patients who
received AC followed by TH [docetaxel/
trastuzumab] had a 51 percent reduction
(Slamon 2005; [1.3]). The conclusion was
that both of the arms were better than the
nontrastuzumab arm, and it was stated
that there weren’t enough data to compare
the two trastuzumab arms. What are your
thoughts regarding these data?
DR WINER: Assuming the hazard ratios
hold up and nothing further shows up in
the data, I’m struck that while TCH clearly
reduced the risk of disease recurrence, a
trend for a better outcome occurred in the
women who received AC followed by TH. It
was said that these are not different from one another; however, I think it is unlikely
that TCH will be better. At the moment, I
would use TCH only for patients in whom
I am particularly concerned about cardiac
toxicity.
I’ll also add that the TCH regimen used in
the BCIRG study is not an easy regimen to
get a patient through, and so apart from
the cardiac issues, I think it’s a more toxic
regimen than AC followed by docetaxel.

Select publications
|
