You are here: Home: Meet The Professors Vol. 3 Issue 6 2005: Case 5

Edited excerpts from the discussion:
DR GOLDBERG: This 70-year-old woman
presented with Stage II breast cancer. She
had an ER/PR-positive, HER2-negative, 2.5-
centimeter adenocarcinoma with two positive
lymph nodes. She received adjuvant CAF
chemotherapy and then went on to tamoxifen
for five years. After completing tamoxifen,
she was put on anastrozole. She’s been
on anastrozole for about a year and she’s
doing well.
DR LOVE: Gersh, can you discuss how
you approach these patients who have
completed five years of tamoxifen in terms
of deciding whether to use an AI and which
one?
DR LOCKER: If their tumors are node-positive,
they should go on an AI, unless they
have severe osteoporosis. I use letrozole
because that was used in the original study,
and I’m trying to be evidence based. I
suspect the other AIs would be just as good.
Data from Europe support anastrozole after
five years of tamoxifen (Jakesz 2005a).
In a woman with node-negative disease, if
there were any negative prognostic features,
such as a large tumor, then I would switch
to letrozole after tamoxifen. I do not
routinely switch breast cancer patients with
T1-B, ER-positive, node-negative tumors.
In that group of women, tamoxifen is as
much a chemopreventative as it is an adjuvant
therapy, and I’m not sure whether the
potential side effects, in terms of bone or
the need for bisphosphonates, merit it. On
the other hand, if the tumor is a T1-C/N0,
I probably would start them on letrozole at
five years.
DR LOVE: How would you manage a patient
who had five or 10 positive nodes initially
and completed adjuvant tamoxifen more
than a year or two ago?
DR LOCKER: I have “sinned” and put
patients with 20 positive nodes on letrozole
five years after completing tamoxifen.
I know I will burn in hell for this, but they
had 20 nodes and I don’t care. For someone
who’s node-negative, I won’t do it that late.
DR LOVE: Incidentally, would you start a
patient with HER2-positive disease and 20
positive nodes on adjuvant trastuzumab five
years later?
DR LOCKER: No, I would not. One of the
things about HER2-positive disease is that
the side effects are bad and can be bad
early. A few patients will probably have a
recurrence late, but I think the greatest
concern is with those who have a recurrence
earlier.
DR LOVE: Would you treat that same patient
with adjuvant trastuzumab two years after
diagnosis?
DR LOCKER: Probably.
DR LOVE: Aman, how do you think exemestane,
letrozole and anastrozole compare
with regard to serious toxicities?
DR BUZDAR: That’s an important issue we
need to discuss with patients. Even though
these studies did not compare one aromatase
inhibitor head-on with another, the
ATAC study, which has the longest follow-up,
has not shown any increase in cardiovascular
events with anastrozole, and substantial
reduction was apparent in cerebrovascular
events (Howell 2005), whereas with the
letrozole in BIG-1-98, an increased risk of
cerebrovascular accidents was apparent and
also an increased risk of fatal myocardial
infarcts (Thürlimann 2005b). These numbers
are small but of some concern. The same thing was seen with the myocardial events
in the exemestane study (Coombes 2004). I
am concerned, but I discuss the data with
the patient and use the aromatase inhibitor
for the setting in which the most data are
available.
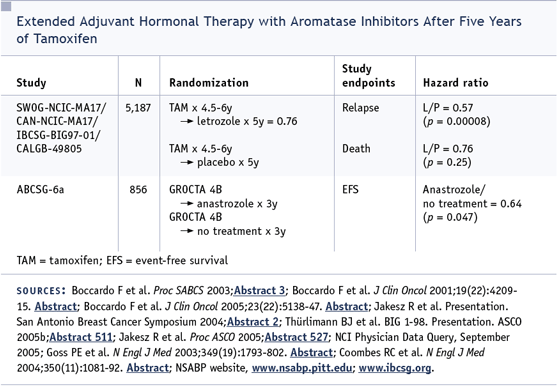
We now have some of the data from the
Austrian study, in which anastrozole was
used after five years of tamoxifen (Jakesz
2005a). We see a similar proportional reduction
in the risk of recurrence, which gives us
one more piece of information that suggests
we may be able to use anastrozole in this
setting.
DR LOVE: At this time, what do you think
is the most defensible aromatase inhibitor
to use initially and after two to three years,
Gersh?
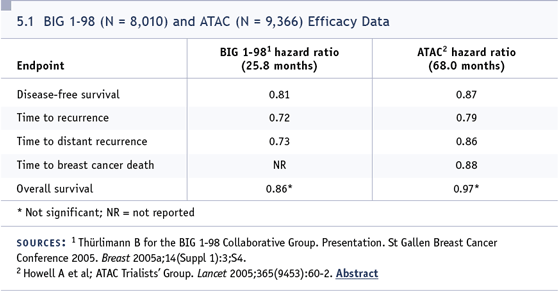
DR LOCKER: Up front, it’s clearly anastrozole.
Until we have five or six years of data
in the BIG I-98 study, anastrozole is the
drug that is most reasonable up front (5.1).
After five years, letrozole should be used. I
have no doubt that anastrozole will probably
be just as good, but I act on the data that
we have.
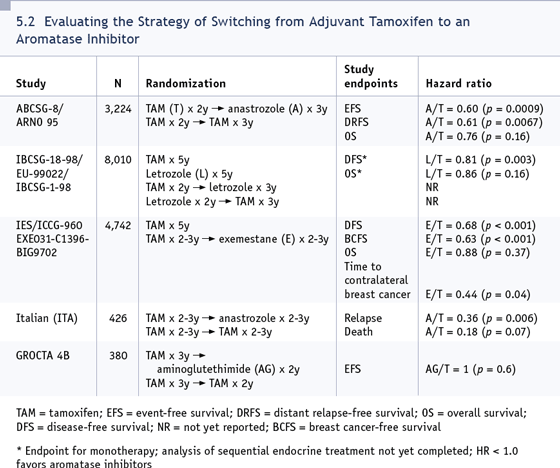
In terms of the switching studies, the IES
data certainly support exemestane, while
the German-Austrian and the Italian data
support anastrozole (Coombes 2004; Jakesz
2005b; Boccardo 2005; [5.2]). I think either
one is acceptable. I would probably use
anastrozole, only because I have more experience with it in the adjuvant setting.
When the BIG I-98 trial reports on the two
switching arms — and I’m praying they are
not underpowered — then we will have an
answer as to whether letrozole should be
used after two years of tamoxifen. However,
for now I go with the most mature studies
that address the specific situations.
DR LOVE: Aman, do you agree with Gershon
as to which AI should be used initially and
when switching after two to three years?
DR BUZDAR: My feeling is that if you look
at the efficacy, either up front or after two
to three years, or even after five years, all
these aromatase inhibitors show similar efficacy.
Differences in safety exist, but you
have to keep in mind that you are looking
across the trials, not at a head-on comparison.
One study is comparing anastrozole
with exemestane, and that will provide
head-on safety data.
However, I totally agree with Gershon that
we have the most mature safety data for
anastrozole, which has the longest followup,
and the efficacy data of BIG-1-98 is
almost a mirror image at the two-year
follow-up. That study, at least, confirms the
ATAC data, in which at two and a half years
we saw a similar proportional reduction in
risk of recurrence.
DR DESAI: When you do switch patients
after two or three years of tamoxifen,
patients ask whether a total of five years of endocrine therapy is enough. They want
to continue therapy. What do we do about
those patients?
DR LOCKER: The switching trials are problematic,
because the implication is that if
you switch, you stop therapy at five years.
Does it make sense that we give five years
of tamoxifen followed by five years of letrozole,
but when we switch, we give only five
years of therapy — two years of tamoxifen
followed by three years of anastrozole or
exemestane?
I don’t know what to do with those patients.
I use the “20-node rule.” If they had a lot of
positive nodes and I’m really worried, then
they’re going to receive hormones until they
die. If they are node-negative, I think you
should just go by what the studies say.
DR BUZDAR: I totally disagree with Gershon
on this point. If you look at the hazard
ratios for patients who have 20 versus zero
positive nodes, the risk of recurrence in the
first two to five years becomes very high
as the number of nodes increases. As time
passes, the risk of recurrence for the two
groups becomes very close. The patient who
has survived five years disease free has a
risk close to that of the patient who had
maybe one or no positive nodes (Saphner
1996).
DR LOCKER: What about looking at receptor
status within that subset? I know it’s a
subset of a subset analysis, but the women
who had 20 positive nodes and are receptor-positive
are not the same as the women who
had 20 positive nodes and are receptor-negative.
DR BUZDAR: The recurrence risk is regardless
of the receptor status. Patients are at
increased risk in the first several years, and
after that, while the risk is there, the differences
between a high number of positive
nodes and negative nodes or a low number
of positive nodes tend to become blurred.
DR LOCKER: Well, having said that,
remember that for a woman with node-positive
disease, from year five to year 10 after
tamoxifen, the absolute yearly risk for recurrence
is two percent per year.
DR LOVE: Do you agree with that, Aman?
DR BUZDAR: It is true that there is a risk
and it is higher than the normal patient
population, but my point is that when
comparing the risk between 20 versus two
positive nodes, the differences become very
close.
DR DRAGON: My understanding in looking
at the trial of letrozole after tamoxifen is
that 20 percent of the recurrences occur
after five years. How does that impact decision-
making for node-negative patients,
where you’re talking about a relatively
small number of women who recur, and then
reducing that by 40 percent? We’re talking
about a one or two percent benefit for five
years of letrozole, which is, by my calculation,
about $14,000 per patient. So you’re
treating 100 women at $14,000 per patient
to reduce, perhaps to eliminate, one or two
recurrences over the next five years. Is that
a rational thought process?
DR LOVE: It is not uncommon to see adjuvant
chemotherapy used under similar
circumstances.
DR LOCKER: Other issues arise with the
patients with node-negative disease in the
MA17 trial. For example, the odd survival
data, showing a statistically significant
survival advantage in patients with nodepositive
disease, but no survival advantage
in women with node-negative disease. In
fact, it’s trending the wrong way. I think Dr
Dragon’s point is very well taken. In patients
with node-negative disease, you have to be
selective as to whom you treat with letrozole
after tamoxifen.
DR SMITH: There seems to be a bit of a
schism between those who would start
patients on anastrozole or letrozole initially
versus those who would give tamoxifen
for two or three years and then switch the
patient to an aromatase inhibitor.
DR BUZDAR: You bring up a very important
point. At MD Anderson, we do not use
tamoxifen up front on any patient who is
postmenopausal. Right now, in 2005, I do
not think we can say that any subset of
postmenopausal patients with ER-positive disease should start on an anti-estrogen and
switch to an aromatase inhibitor after two
or five years. I think the data we have are
very convincing that starting with an aromatase
inhibitor is better. It reduces the risk of
recurrence and the overall safety profile of
the therapy is better. I think the question is
whether a subset exists in which it is better
to start on tamoxifen and then switch later.
This is a research question and the studies
are ongoing.
DR LOVE: Gersh, when deciding on an adjuvant
therapy, how much of an issue is the
risk of endometrial cancer and thrombosis?
DR LOCKER: I remember sitting with Aman
when they presented the ATAC hysterectomy
data, which was a curveball. It showed
that seven percent of women on tamoxifen
underwent a hysterectomy during five years
of treatment, compared to a couple percent,
at the most, for the patients taking anastrozole.
I think, in terms of the switching
versus starting, that is a number you cannot
escape. One out of every 100 women who
receives tamoxifen for two or two and a half
years and then switches to anastrozole will
recur and presumably die, who would not
have if they were on anastrozole initially.
What do you say to that one patient? I
don’t think anybody can predict who that
one woman is, such that you can start her
on anastrozole and start everybody else on
tamoxifen.
DR LOVE: Aman, do you think we will see a
survival advantage for aromatase inhibitors
over tamoxifen?
DR BUZDAR: I think that you will see a
survival advantage once these studies
mature. I think if you did a meta-analysis of
the data from the current studies, you would
see a significant survival advantage.
I just want to remind some of you that
initially when tamoxifen was being evaluated,
only one or two trials showed a
survival advantage.
It was the first Oxford
meta-analysis that showed a dramatic reduction
in the risk of death because you need
a lot of events. In these postmenopausal
patients with ER-positive disease, it takes
much longer for the events that contribute
to the survival advantage to develop. Major
competing causes of death play a role and
breast cancer becomes a secondary cause
of death, so you need a large number of
patients and longer follow-up.
DR STEINECKER: Should we be a little
more attuned to following lipid profiles in
patients on aromatase inhibitors?
DR BUZDAR: It would be reasonable — at
least with letrozole and exemestane, for
which we have data indicating that those
agents change the lipid profile adversely
in a sizable number of patients — to have
baseline information and to re-evaluate the
lipids several months down the line. If they
are being affected adversely, then maybe
an appropriate intervention should be made
before any major event related to that effect
develops.
I would like to come back to this issue of
cost. Patients don’t come to us because they
want us to manage their financial affairs.
They are coming to receive the best treatment
for their cancer. We need to tell them
what we think is the best and most effective
treatment and then if the cost is an issue,
we need to help them. Some of the patients
who bring up the issue of cost have a bag
full of other, alternative “health medications”
for which they may be paying twice
as much per month as the difference in
costs between these two drugs.
Select publications
|
